"It's a paradigm shift." This is how Prof. Ulf Teichgräber, Director of the Institute of Diagnostic and Interventional Radiology at the University Hospital Jena, describes the improvement in communication with sponsors and CROs through the use of mint Lesion™. Together, Ms. Laura Graziani, study coordinator, Ms. Elisabeth Lammers, study assistant/MTRA, and Prof. Teichgräber describe their journey towards a successful clinical trial center that can now manage up to 40 clinical trials simultaneously.
Where results previously had to be painstakingly entered into Excel spreadsheets, mint Lesion™ has taken over the evaluation of all findings since its introduction in 2015, thus increasing the objectivity and validity of the study results.
Workflow optimization, increased efficiency and reduced errors in clinical trials
Related Resources
Related Resources
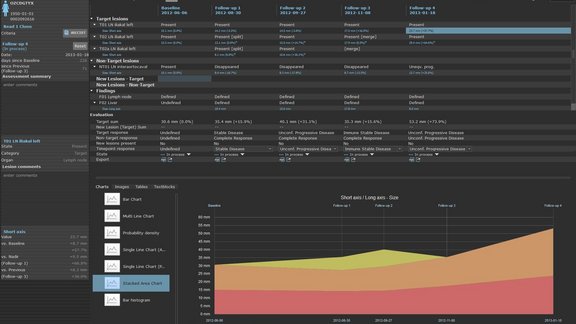
New iRECIST Oncologic Response Criteria already in clinical use at the University Medical Center – Tübingen, Germany
Shortly following the release of the new iRECIST- oncologic response criteria (Lancet Oncology, March 2017) the University Medical Center in Tübingen,…

Mrs. Kelie Luby joins Mint Medical's team
Mrs. Kelie Luby will join Mint Medical’s team from January 2017 as new Vice President Clinical Trials Software and will be introduced at the upcoming…
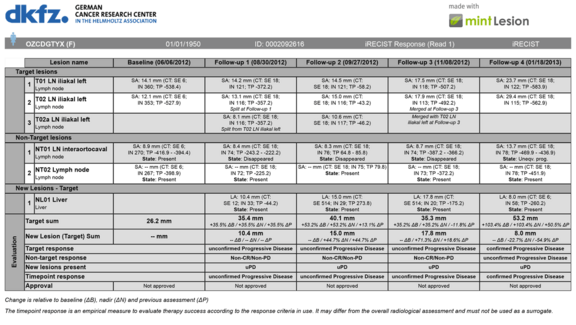
Novel oncologic therapy response criteria (iRECIST and more)
Advances in oncology therapies, such as novel immune-related agents, require adapted and refined criteria and guidelines for the assessment and…
