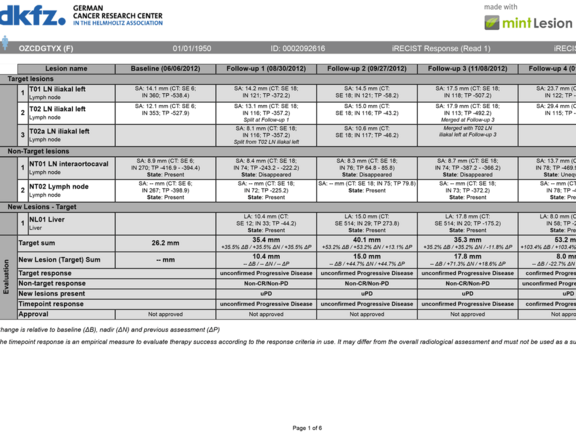
Advances in oncology therapies, such as novel immune-related agents, require adapted and refined criteria and guidelines for the assessment and prediction of treatment response.
At RSNA 2016, Mint Medical showcases a comprehensive set of new criteria including mRECIST mesothelioma, the Lugano (Cheson 2014 lymphoma) Classification, the Prostate Cancer Working Group (PCWG2) criteria, and iRECIST.
As an example, immune-related therapies can show tumor growth from treatment effect rather than true disease progression (“pseudoprogression”). mint Lesion™ 3.3 now supports the iRECIST criteria as currently drafted by the RECIST working group. iRECIST is the first immune-related criteria distinguishing between an unconfirmed and a confirmed Progressive Disease (PD).