Optimize your oncology clinical trial evaluations with mint REX
In the evolving world of clinical trials, accuracy, compliance, and data integrity are paramount. mint REX addresses key challenges by offering a simple and standardized tool for response assessment.
Built as a powerful, hands-on Microsoft Excel add-in, mint REX streamlines oncology response assessment by enhancing accuracy, minimizing data errors, and reducing queries. It enables effortless data management and precise disease classification, driving efficiency from day one.
Looking for a smarter way to manage tumor response assessment data with confidence?
Oncology clinical research empowered by mint REX
Unlock the full potential of your oncology clinical trials with a purpose-built tool that elevates accuracy, ensures criteria compliance and accelerates efficiency, delivering measurable value for every stakeholder
Enhanced Data Entry and Accuracy: Ensure criteria conformity and perform data validation with automatic checks that highlight and explain any rule violations or discrepancies.
Fewer Data Queries and Corrections: Reduce the need for manual queries and corrections to streamline the review process.
Seamless Data Verification: Easily verify source data assessments against EDC entries for improved consistency and accuracy.
Simplified Lesion Tracking: Effortlessly track longitudinal tumor response evaluations across visits.
Automated Response Calculation: Derive Overall Responses (CR, PR, SD, PD) and disease compartment responses (Target, Non-Target, New Lesions) automatically for faster, more accurate assessments.
Supported response criteria
RECIST 1.1 evaluation criteria for solid tumors
Based on the publication of Eisenhauer EA, et al. 2009
iRECIST (with RECIST 1.1) evaluation criteria for
use in trials testing immunotherapeutics
Based on the publication of Seymour L, et al. 2017
PCWG3 (with RECIST 1.1) evaluation criteria for castration-resistant prostate cancer
Based on the publications of Scher HI, et al. 2016
RANO HGG evaluation criteria for high-grade gliomas
Based on the publication of Wen PY, et al. 2010
Lugano evaluation criteria for Hodgkin and non-Hodgkin lymphoma
Based on the publication of Cheson BD, et al. 2014
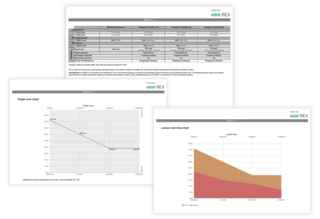
Clear and comprehensive reports
Protocol and Subject ID: Key identifiers for accurate tracking.
Timepoint Names and Visit Dates: Detailed timelines of patient visits.
Lesion Measurements, Locations, and Descriptions: Precise data for each lesion.
Response Assessments: Comprehensive evaluation of Target, Non-Target, and New Lesions.
Overall Visit Response: Aggregated summary across all follow-up timepoints.
Lesion Overview Chart: Visual representation of lesion-level data.
Target Sum Chart: Graphical view of total lesion measurements over time.
Optional Signature Page: Validation and attestation for enhanced compliance.
mint REX seamlessly integrates into diverse workflows, adapting to the unique needs of different departments and roles. It empowers study teams at investigational sites, CROs, and sponsors to master response criteria with ease.
By uniting all stakeholders through a single, efficient, and user-friendly tool, mint REX delivers a scalable, low-maintenance solution specifically tailored for oncology clinical research.
The result?
Deliver faster, more accurate oncology response assessments — reducing data corrections and empowering teams with clear, actionable reports.