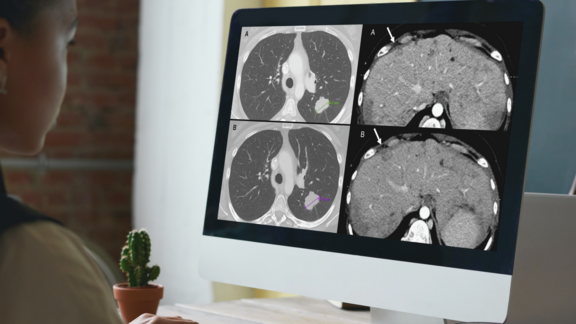
Emily Ferris and Alex Arbuckle describe how using mint Lesion™ has enhanced their clinical trial operations, enabling “a more cooperative approach between [their] radiologists and oncologists” while simultaneously saving time and reducing costs.
Faced with a multitude of clinical trial site reads each day, Ms. Ferris and Mr. Arbuckle explain how they benefit from a more efficient workflow by using a dedicated software platform which provides a comprehensive and compliant response assessment and supports them in maintaining oversight of clinical trial imaging analysis design.
Ms. Ferris is a Lead Image Analyst and Mr. Arbuckle is a Clinical Research Imaging Core Manager at the University of Wisconsin Carbone Cancer Center.
Click here or on the image above to watch the full video on YouTube.