A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
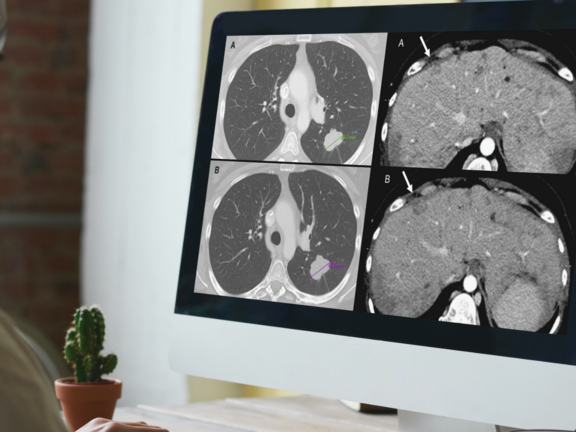
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources
Early lung cancer detection through LDCT screening as a potential way to improve patient outcomes in Germany
The establishment of a nation-wide lung cancer screening program in Germany has been a subject of considerable interest and debate among healthcare…

RACOON FHIR Workshop: Empowering Healthcare Research via Enhanced Interoperability
This week Brainlab hosted the RACOON FHIR Workshop with around 40 participants. The workshop was organized by Mint Medical and supported by Snke OS,…
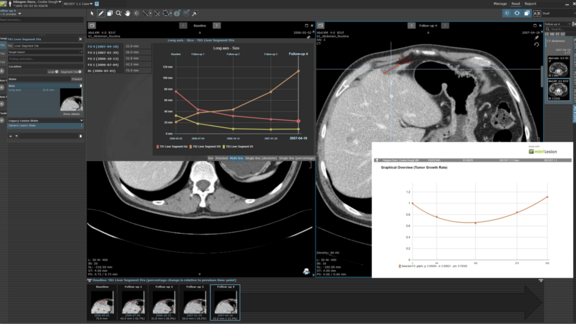
Tumor Growth Rate Modeling: A Novel Approach to Evaluating the Efficacy of Cancer Therapies
The 2020 review of Clinical Trial Evidence Supporting US Food and Drug Administrative Approval of Novel Cancer Therapies Between 2000 and 2016…