A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
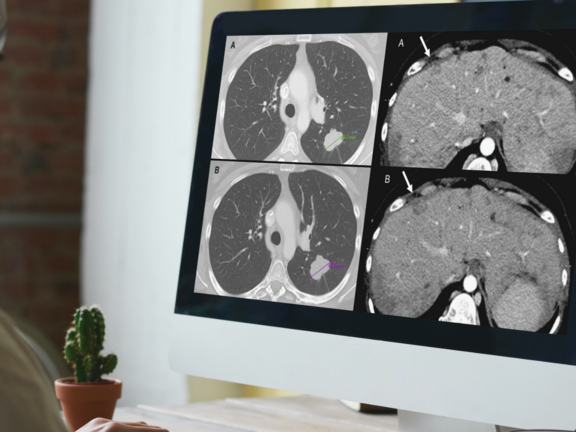
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources
AI in Radiology: Bridging the Gap Between Integration Challenges and Untapped Potential
The influence of artificial intelligence (AI) on the field of radiology has substantially increased in the last years. Today, AI can be applied to…
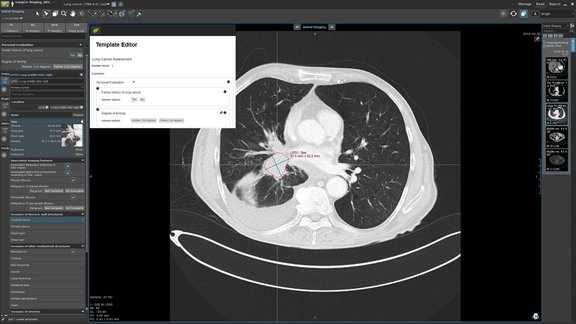
Customizing structured data collection with mint Lesion Template Designer
We are thrilled to announce a forthcoming addition to mint Lesion: the Template Designer. This feature will transform the way users interact with our…

Improving Cancer Care Through Standardization: Interview with Prof. Thomas Kröncke on the BZKF Project BORN
The Bavaria-wide oncological radiology network (BORN) Project was launched on August 1, 2022, with the objective of improving cancer care in Bavaria…