A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
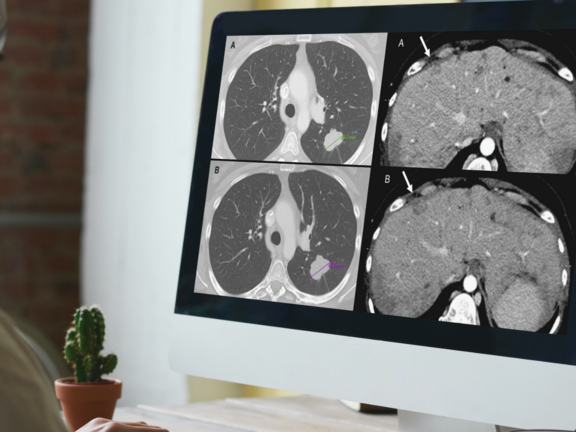
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources

RACOON: A revolutionary project with all German university hospitals in the University Medicine Network (NUM)
The idea of aggregating clinical data from different sources, processing it in a structured way and using it for joint research may not be new, but…
High-quality annotated data in clinical routine
The desire to standardize reporting and to get even more out of their oncological images led to the partnership of the Noordwest Ziekenhuisgroep in…
Raising our awareness in pediatric cancer
The terms rare, uncommon, infrequent are commonly used to describe the occurrence of cancer in children – from a prevalence perspective, “only”…