The terms rare, uncommon, infrequent are commonly used to describe the occurrence of cancer in children – from a prevalence perspective, “only” around 400,000 children are diagnosed with cancer each year. However, for parents and children facing a cancer diagnosis, rare correlates to the isolation of being thrust into unknown terrain, learning a new language of medical terminology, and understanding and trying to navigate treatment recommendations. They are suddenly cast into an array of hospital stays that sometimes last months, with appointments, procedures, and medications but with no clear roadmap on how to do this.
While statistically, survival rates have improved, many of the current treatment approaches are decades old with consequential treatment effects that endure long after the end of therapy. Childhood cancer needs the collective power of industry frameworks, funding, research, and innovation to make the same advances we have made in other areas of cancer research.
In June of 2020, a series of papers published in The Lancet Oncology met the important goal of standardizing imaging guidelines and response assessment recommendations for clinical trials as part of the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Covering pediatric low-grade glioma, high grade glioma and diffuse intrinsic pontine glioma (DIPG), these important publications provide a path to standardization of imaging and response evaluation that will support the critical need of outcome comparison across clinical trials [1, 2, 3].
We at Mint Medical followed this lead by developing radiology and clinical data assessment templates in mint Lesion™ to support the standardized evaluation and structured data reporting needed for pediatric clinical trials in low-grade glioma and high-grade glioma. We commit to do the same in DIPG and neuroblastoma. Evidence-based medicine is supported by the ability to compare outcomes. In rare indications, the importance of comparability is even more imperative.
While these response assessment criteria require the application of diameter-based 2D measurements, volumetric measurements provide further information to guide evidence-based research, as a study with mint Lesion™ has shown [4], and validate the optimal evaluation measures. Understanding that structured, mineable, semantic data is essential, we provide both with mint Lesion™ in a single framework that meets the structured data reporting needs for current clinical research but also guides further information on optimal approaches in treating childhood cancers. At Mint Medical, we are dedicated to doing our part in contributing to advance childhood cancer research and providing better support for children and their families.
[1] Fangusaro J, Witt O, Hernáiz Driever, P, et. al., Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020 Jun;21(6):e305-e316. doi: 10.1016/S1470-2045(20)30064-4. Erratum in: Lancet Oncol. 2020 Dec;21(12):e553. PMID: 32502457.
[2] Erker C, Tamrazu, B, Poussaint TY, et. al., Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020 Jun;21(6):e317-e329. doi: 10.1016/S1470-2045(20)30173-X. Erratum in: Lancet Oncol. 2020 Aug;21(8):e372. PMID: 32502458.
[3] Cooney TM, Cohen KJ, Guimares CV, et. al., Response assessment in diffuse intrinsic pontine glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020 Jun;21(6):e330-e336. doi: 10.1016/S1470-2045(20)30166-2. Erratum in: Lancet Oncol. 2020 Aug;21(8):e372. PMID: 32502459.
[4] L.A. Gilligan, M.D. DeWire-Schottmiller, M. Fouladi, P. DeBlank, J.L. Leach, Tumor Response Assessment in Diffuse Intrinsic Pontine Glioma: Comparison of Semiautomated Volumetric, Semiautomated Linear, and Manual Linear Tumor Measurement Strategies. American Journal of Neuroradiology May 2020, 41 (5) 866-873; DOI: 10.3174/ajnr.A6555
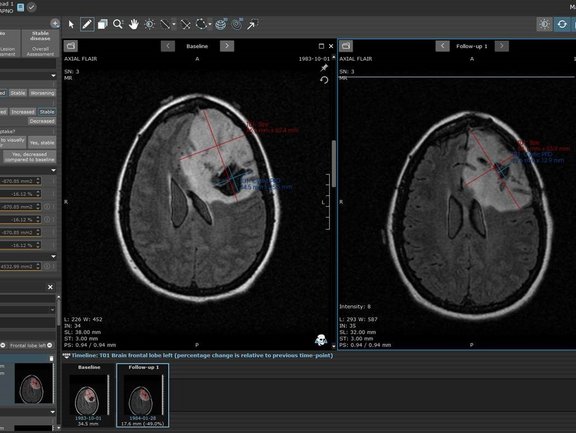
Raising our awareness in pediatric cancer
Related Resources
Related Resources
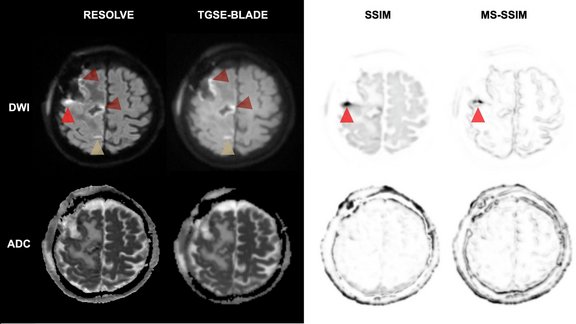
Improving Postoperative Brain Tumor MRI: TGSE-BLADE vs. RESOLVE DWI
Early MRI scans are critical for patients who have just undergone brain tumor resection to evaluate the surgery's success and plan future treatments.…

Centralized Management for Clinical Trial Communications and Workflows
Requests for radiological clinical trial evaluations frequently rely on fragmented methods such as emails, phone calls, and spreadsheets. This…
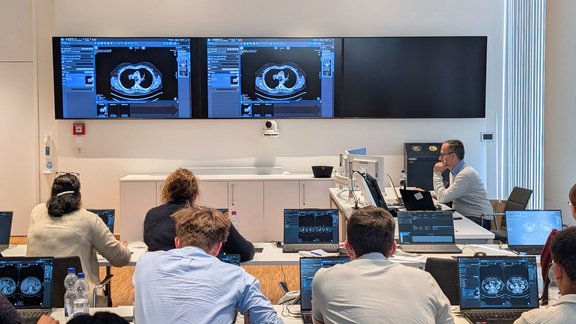
Successful “RECIST and Beyond” Workshop in Cologne: Advancing Precision in Oncologic Imaging
How can complex tumor findings be assessed accurately, reproducibly, and in line with clinical guidelines?