A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
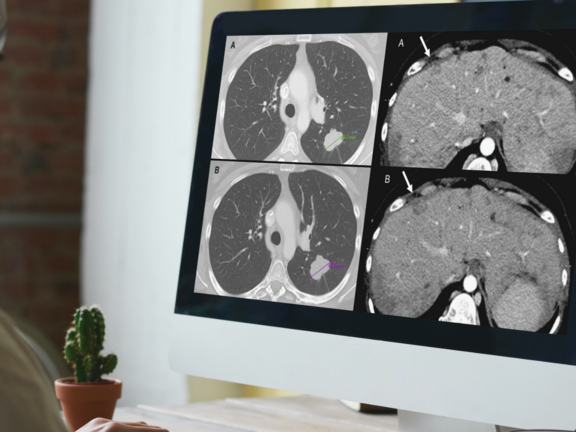
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources

Leave No Data Behind: Commitment to Data Excellence
At Mint Medical, we understand data as a cornerstone for improving patient care and groundbreaking research. Our mission is clear: Leave No Data…

Transform Your Research with the Template Designer
At Mint Medical, we know that organized and high-quality data is the foundation of successful research. That's why we've developed the Template…

ESOI-EORTC Workshop: Hands-On Training in Assessing Tumor Response to Treatment
The ESOI-EORTC workshop, hosted by the European Society of Oncological Imaging (ESOI) and the European Organization for Research and Treatment of…