Categories:
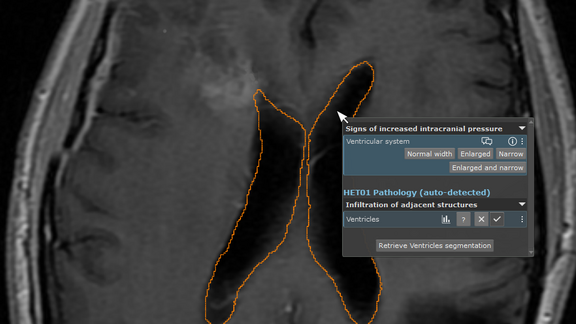
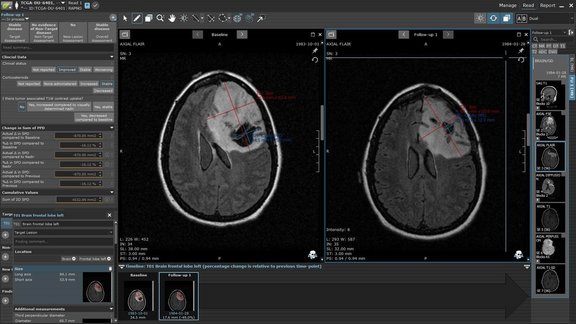
„Anatomical GPS“ for mint Lesion™: Leveraging the Snke OS/Brainlab Anatomic Patient Model to drive anatomic-context-awareness and automation within mint Lesion™
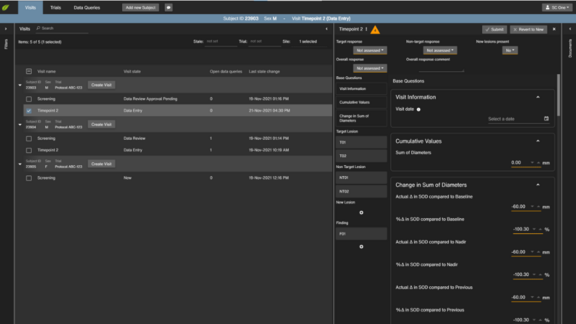
More automation within mint Lesion™? Automated context-dependent template selection, filtering of relevant questions or even fully automated organ and…