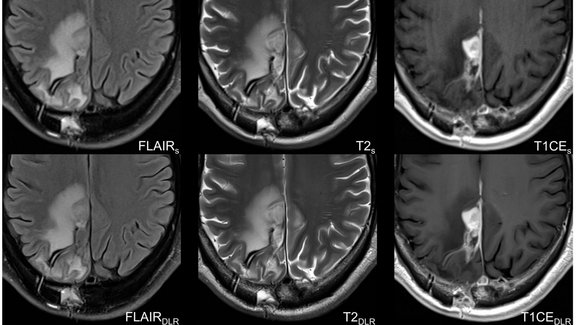
"It's a paradigm shift." This is how Prof. Ulf Teichgräber, Director of the Institute of Diagnostic and Interventional Radiology at the University Hospital Jena, describes the improvement in communication with sponsors and CROs through the use of mint Lesion™. Together, Ms. Laura Graziani, study coordinator, Ms. Elisabeth Lammers, study assistant/MTRA, and Prof. Teichgräber describe their journey towards a successful clinical trial center that can now manage up to 40 clinical trials simultaneously.
Where results previously had to be painstakingly entered into Excel spreadsheets, mint Lesion™ has taken over the evaluation of all findings since its introduction in 2015, thus increasing the objectivity and validity of the study results.
Click here or on the image above to watch the full video on YouTube.