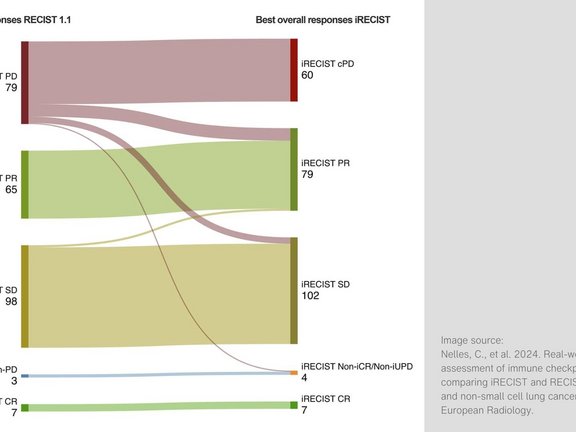
A retrospective study conducted at University Hospital Cologne compared two criteria for assessing therapeutic response to immunotherapy: iRECIST and RECIST 1.1. The comparison focused on a group of patients with melanoma or non-small cell lung cancer (NSCLC) who received immune checkpoint inhibitors.
The study aimed to determine which method is better suited to capture atypical response patterns to immunotherapy in clinical practice. The study included 252 patients (150 men and 102 women) with melanoma or NSCLC.
RECIST 1.1 is the established standard for assessing treatment response in solid tumors in clinical trials; however, it has limitations when applied to immunotherapies, as it does not account for atypical response patterns like pseudoprogression. Pseudoprogression describes a phenomenon in which tumors initially increase in size before responding to treatment. iRECIST was specifically developed to address this challenge by better capturing atypical responses, including pseudoprogression.
Key findings of the study include:
- Among the patients who showed disease progression (PD) according to RECIST 1.1, iRECIST did not confirm progression in 33.6% of cases.
- iRECIST demonstrated a higher overall response rate (ORR) of 34.1% compared to 28.5% with RECIST 1.1. Additionally, the disease control rate (DCR) was higher: 74.6% with iRECIST versus 67.4% with RECIST 1.1.
- Time to progression (TTP) was significantly longer when using iRECIST for response assessment, with an average TTP of 618.3 days compared to 538.1 days for RECIST 1.1.
These results indicate that iRECIST is more effective in capturing atypical responses to immunotherapy, particularly in patients with pseudoprogression. This may help prevent patients from being prematurely classified as non-responders and discontinuing effective treatment early. Future studies should prospectively investigate whether the ability to identify such response patterns outweighs the potential downside of delayed progression diagnosis due to the need for follow-up imaging to confirm disease status.
Adopting iRECIST in clinical practice could help improve immunotherapy outcomes by enabling more accurate radiological assessments of therapeutic response.
Read the original publication here.
Nelles, Christian, et al. 2024. „Real-world response assessment of immune checkpoint inhibition: comparing iRECIST and RECIST 1.1 in melanoma and non-small cell lung cancer patients.” European Radiology.