This research study [1] aimed to compare the feasibility and reliability of manual versus software-assisted assessments of computed tomography (CT) scans according to iRECIST (immune Response Evaluation Criteria in Solid Tumors) in oncological patients undergoing immune-based treatment. The study involved evaluating CT scans of 30 tumor patients at baseline (BL) and two follow-ups (FU1 and FU2), resulting in 360 tumor assessments. After image interpretation, tumor burden and response status were calculated manually or with software assistance.
mint Lesion™ was employed for the software-assisted assessment. Target and non-target lesions were initially annotated within the software and assessed using dedicated tools. The software facilitated adherence to the iRECIST guidelines by offering assessment guidance and highlighting deviations or errors (such as one-dimensional measurement of lymph nodes or an excessive number of lesions).
The reading time, error rate, and inter-reader agreement were compared between the manual and software-assisted approaches, with the following findings:
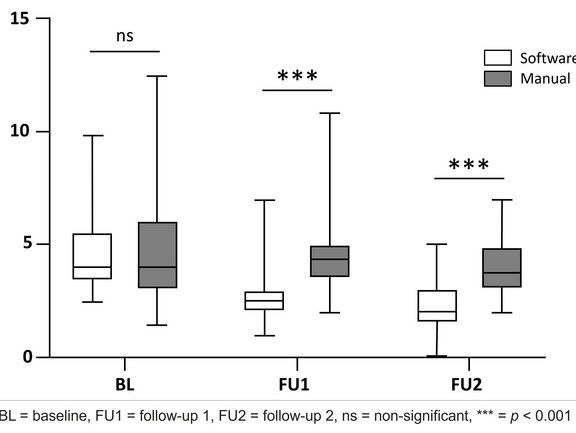
- Reading Time: The study found significantly shorter reading times for software-assisted assessments compared to manual assessments at both follow-ups. The median reading time at FU1 was 2.5 minutes for the software-assisted assessment and 4 minutes for the manual assessment, and for FU2, it was 2 minutes vs. 3.75 minutes, respectively.
- Error Rate: Software-assisted assessments demonstrated lower error rates, with 0% errors for software-assisted assessments compared to 3.3% for manual assessments at FU1 and 1.7% versus 10% at FU2.
- Inter-Reader Agreement: The study demonstrated good to excellent inter-reader agreement for both manual and software-assisted assessments. However, the agreement was higher for software-assisted assessments, particularly at the second follow-up. The manual assessments' intraclass correlation coefficient (ICC) was 0.91 at FU1 and 0.75 at FU2. For software-assisted assessments, the ICC was 0.93 at FU1 and 0.86 at FU2.
"Our results reveal that software-assisted iRECIST readings can be performed with (I) less time effort, (II) fewer incorrect response assessments, and (III) a higher inter-reader agreement compared to manual approaches," concluded the researchers.
The study's findings demonstrate that utilizing software-assisted solutions is preferable over manual approaches for optimal oncological response evaluation. This is particularly relevant for iRECIST assessments, where accurate evaluation necessitates a solid understanding of the fundamental rules and a comprehensive review of prior examinations, notably streamlined by software assistance.
Read the original publication here.
[1] Ristow I, Well L, Wiese NJ, Warncke M, Tintelnot J, Karimzadeh A, Koehler D, Adam G, Bannas P, Sauer M. Tumor Response Evaluation Using iRECIST: Feasibility and Reliability of Manual Versus Software-Assisted Assessments. Cancers. 2024