The 2020 review of Clinical Trial Evidence Supporting US Food and Drug Administrative Approval of Novel Cancer Therapies Between 2000 and 2016 revealed that although novel cancer therapies showed significant tumor response, the increase in median overall survival was only around two months [1]. This finding raised such questions as whether the current methods of measuring treatment efficacy include scientific evidence from imaging biomarker research; and whether the methods used to measure efficacy in anti-cancer treatment, such as response criteria guidelines and diameter-based measurements, are the most effective tools.
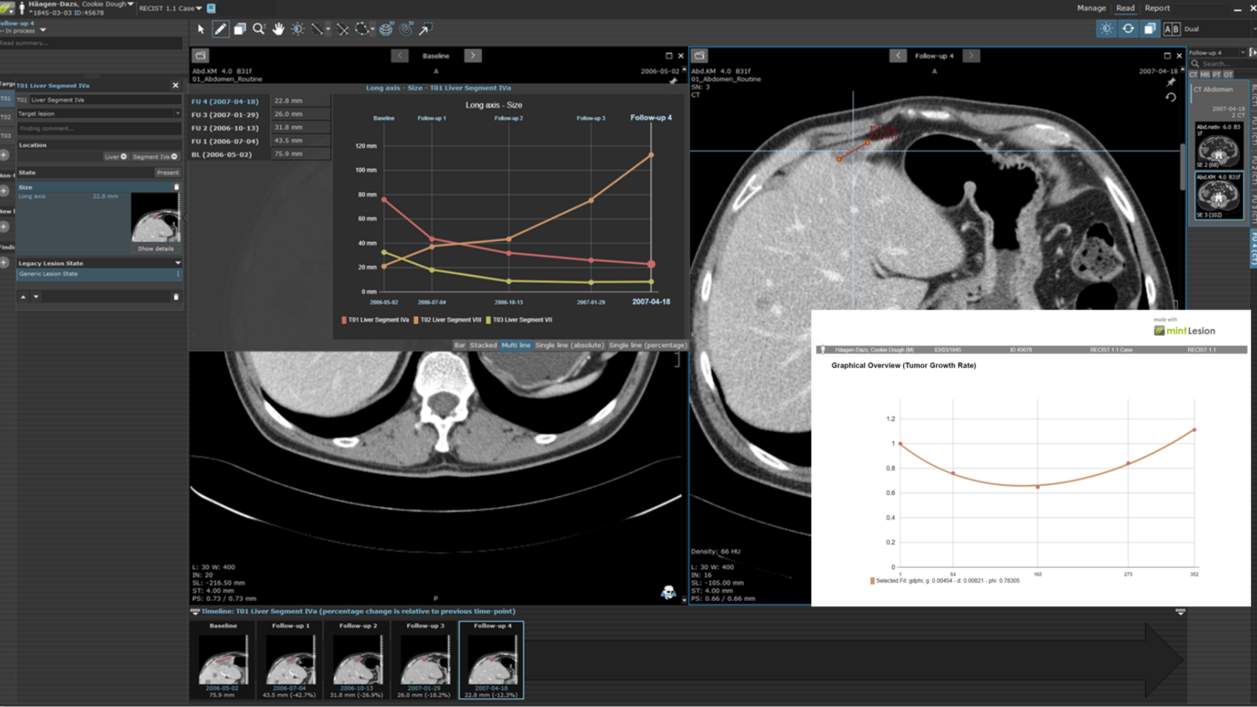
Tumor Growth Rate Modeling (TGRM) could be an alternative approach that utilizes the established standard of a longitudinal evaluation of tumor burden on imaging but has the potential to offer earlier and more reliable signals of efficacy, even when working with smaller datasets. By leveraging TGRM, researchers can refine their understanding of tumor growth patterns and make more accurate predictions regarding treatment efficacy. While computational modeling for evaluating tumor quantity changes across timepoints is not an entirely new concept, these methodologies, while providing essential and valuable information, have not shown notable improvement over imaging-based endpoints with response criteria evaluation. However, recent research has successfully demonstrated an approach with relatively simple computational modeling that can utilize measurement of tumor quantity on imaging with time as a variable [2][3][4][5].
Tumor Growth Rate Modeling Methodology
The proposed Tumor Growth Rate Models utilize mathematical expressions to measure changes in tumor burden as measured on imaging evaluations during a given treatment providing two key values:
- the rate of growth/regrowth g
- the rate of regression/decay d
This approach is based on the concept that the tumor burden changes over the course of treatment are based on two independent processes that occur concurrently: firstly, decay of tumor cells sensitive to treatment and secondly, growth of cancer cells that show treatment resistance. Depending on the fraction of the tumor cells (in)sensitive to treatment, one of three growth patterns can be observed in longitudinal imaging: steady increase of tumor size, steady decrease of tumor size, or a decrease followed by an increase. For both decay and increase, an exponential growth pattern is assumed, leading to the four equations with two different equations for the decay-regrowth pattern.
Unlike traditional approaches that rely on fixed imaging intervals, these equations consider time as a variable, removing the constraint of predefined imaging timepoints. Each participant's data may be fitted to a specific model at a particular time, depending on the number of timepoints available. Each model requires a minimum number of timepoints to accurately estimate the g and d values. Typically, this includes the baseline measurement and two additional follow-up timepoints. One fit model (gdΦ) is optimized for four total timepoints, and in addition to the g and d estimates, also provides a value Φ, which represents the fraction of tumor showing regression. The same methodologies currently utilized in serial assessment of longitudinally acquired participant images can be applied to tumor growth rate models.
While there is potential for this methodology to provide significant value in cancer therapeutic research, the use of this approach as a validated and accepted imaging biomarker in clinical trials is still required. That is, there remains extensive research and validation efforts to be done such that this methodology might supplement the established clinical trial endpoints currently employed with imaging.
Contact us to learn more about TGRM and how this methodology can be applied in mint Lesion™.
[1] Ladanie A, et.al., Clinical Trial Evidence Supporting US Food and Drug Administration Approval of Novel Cancer Therapies Between 2000 and 2016. JAMA Netw Open. 2020 Nov 2;3(11):e2024406
[2] Maitland ML, et.al., Enhanced Detection of Treatment Effects on Metastatic Colorectal Cancer with Volumetric CT Measurements for Tumor Burden Growth Rate Evaluation. Clin Cancer Res. 2020 Dec 15;26(24):6464-6474.
[3] Yeh, C et.al., Tumor Growth Rate Informs Treatment Efficacy in Metastatic Pancreatic Adenocarcinoma: Application of a Growth and Regression Model to Pivotal Trial and Real-World Data, The Oncologist, Volume 28, Issue 2, February 2023, Pages 139–148.
[4] Stein WD, et.al., Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncologist. 2008 Oct;13(10):1046-54. doi: 10.1634/theoncologist.2008-0075.
[5] Wilkerson J, et. al., Estimation of tumour regression and growth rates during treatment in patients with advanced prostate cancer: a retrospective analysis. Lancet Oncol. 2017 Jan;18(1):143-154.