A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
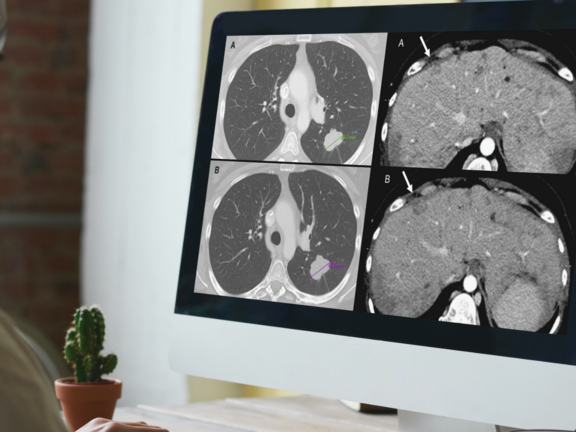
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources

Optimizing Glioblastoma Imaging: Enhancing MRI Efficiency and Quality with Deep Learning
This study investigates the use of deep learning (DL) to optimize MRI protocols for glioblastoma patients, aiming to reduce scan time and improve…
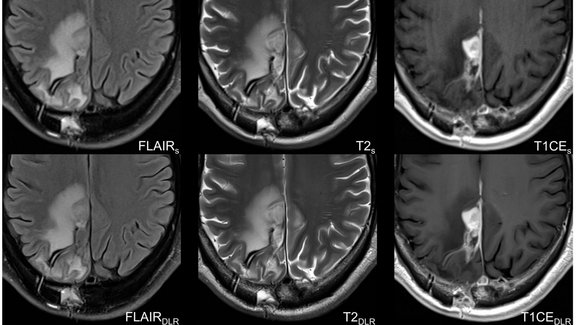
University Hospital Tübingen: Advancing MRI Efficiency in Glioblastoma Care with Deep Learning
This study explores the use of deep learning (DL) to optimize MRI protocols for glioblastoma patients. Glioblastomas, known for being the most…

Insights into the BORN Project: Development and Successes with Dr. Maurice Heimer
The BORN-project of the Bavarian Center for Cancer Research (BZKF) is making swift progress in its second funding phase. A key aspect of the project…