A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
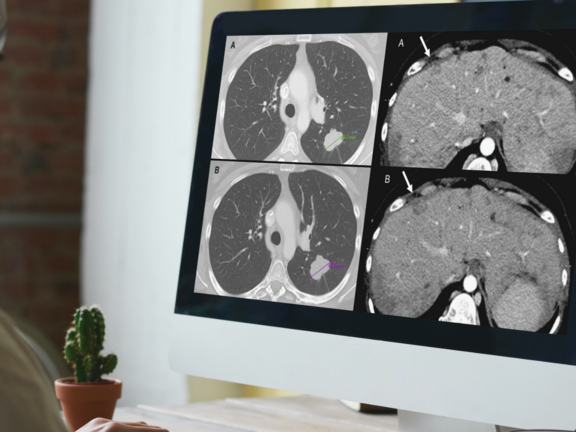
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources

Study Highlights the Potential of Radiomics in Medical Intervention
A recent study conducted by the Medical University of Innsbruck highlights the potential of radiomics in exploring the impacts of Radiochemotherapy…
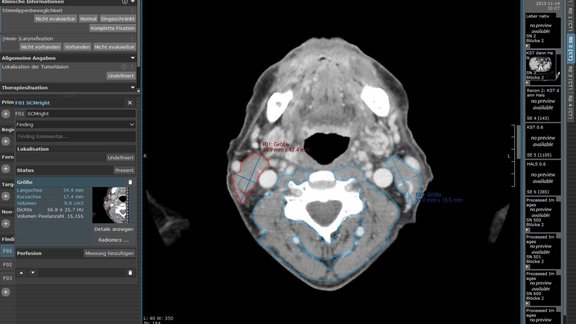
Medical University of Innsbruck: Radiomic Assessment of Radiation-Induced Alterations of Skeletal Muscle Composition in Head and Neck Squamous Cell Carcinoma within the Currently Clinically Defined Optimal Time Window for Salvage Surgery
This study [1] aimed to examine tissue changes in patients with head and neck squamous cell carcinoma (HNSCC) after primary radiochemotherapy (RCT)…
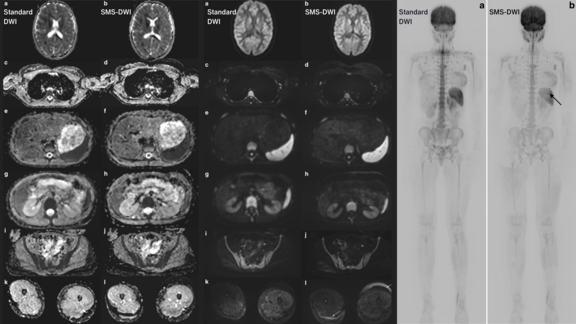
New Technique Speeds Up Whole-Body MRI for Children Without Sacrificing Image Quality, Study Finds
As we mark Childhood Cancer Awareness Month, we would like to highlight the progress achieved in the realm of cancer diagnostics. A recent study led…