A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
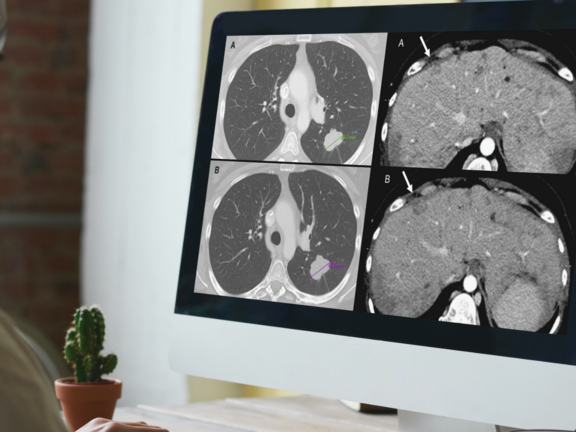
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources
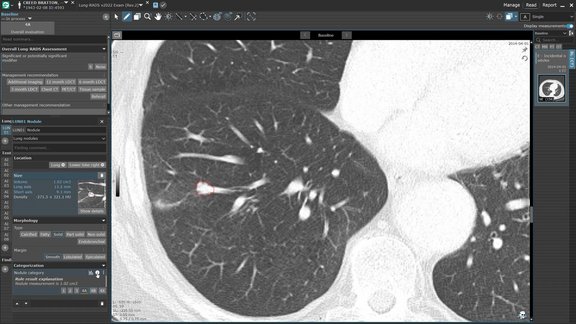
Leverage Advanced AI-Driven Nodule Detection and Analysis for Comprehensive Patient Care
Discover the power of streamlined AI-driven lung screening with contextflow ADVANCE Chest CT integrated into mint Lesion. With automated lung nodule…
Comparison of iRECIST and RECIST 1.1 for Evaluating Immunotherapy in Melanoma and Non-Small Cell Lung Cancer
In a retrospective study conducted at the University Hospital Cologne, the radiological criteria iRECIST and RECIST 1.1 were compared for assessing…

University Hospital Cologne: Comparison of iRECIST and RECIST 1.1 for Evaluating Immunotherapy in Melanoma and Non-Small Cell Lung Cancer
A retrospective study conducted at University Hospital Cologne compared two criteria for assessing therapeutic response to immunotherapy: iRECIST and…