A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
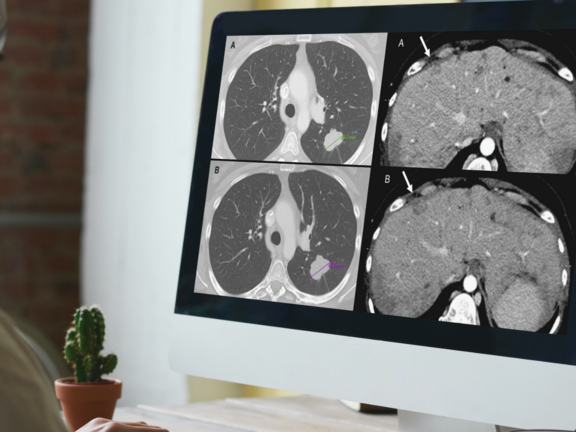
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources

Mint in 30 Minutes - How to adopt radiomics research, clinical trial management, and cognitive assistance in clinical routine
During this year’s virtual ECR 2020, we provided an insight into data-driven radiology with our Mint in 30 Minutes webcast. Using live software…

ExploreCOVID: An explorative cohort study to identify optimal CT imaging biomarkers in combination with clinical markers and PCR-RT for the diagnosis and therapy response assessment of COVID-19
Funded by the German Federal Ministry of Education and Research, the ExploreCOVID project aims to analyze patient history and clinical as well as…

The challenges of TNM-staging for head and neck tumors - Interview with Prof. Dr. Beer
Next to clinical examination and endoscopy, cross-sectional imaging plays an important role in the diagnosis of head and neck tumors. Moreover, the…