A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
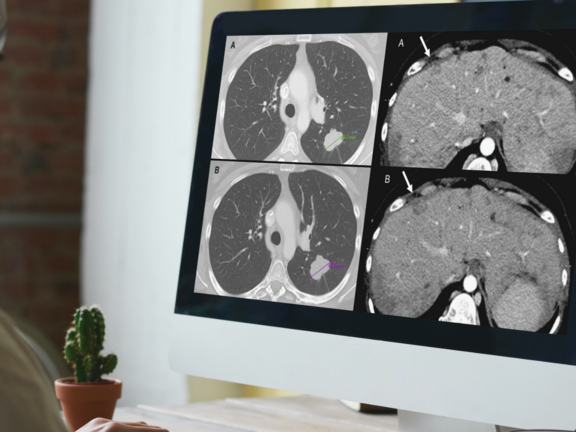
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources

Transforming Oncology Patient Care
Innovative Approach to Structured Routine Reporting with mint Lesion at the University Hospital Zurich Heidelberg, DE, 05/09/2023 - Mint Medical…
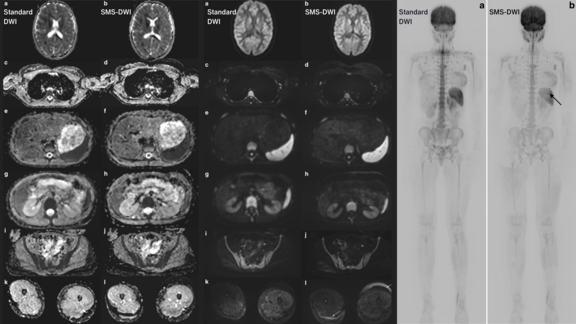
University Hospital Jena: Research Investigating the Viability of Accelerating Whole-Body MRI in Children and Adolescents through STIR DWI with Simultaneous Multi-Slice Excitation
This study[1] addressed the challenges of conducting WB-MRI in paediatric patients, particularly the prolonged acquisition time required for…
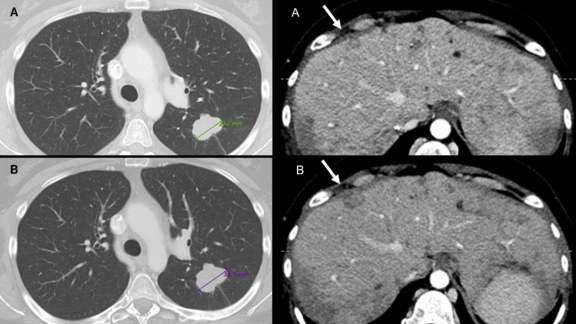
WUSTL: Discrepant Assessments of Progressive Disease in Clinical Trials between Routine Clinical Reads and Formal RECIST 1.1 Interpretations
In this retrospective study utilizing the mint Lesion™ software[1], researchers found that routine clinical interpretations frequently resulted in the…