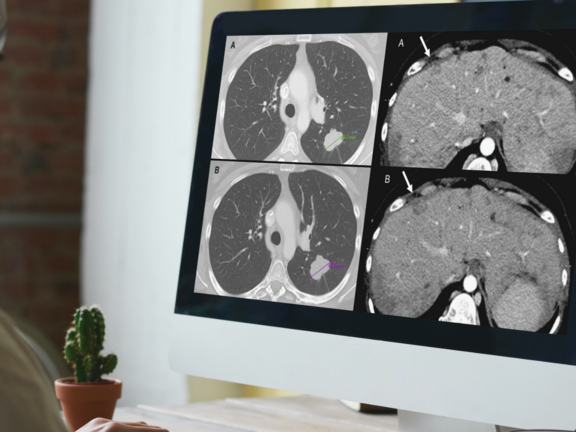
A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources

Developing Standardized Reporting Templates for Sarcomas: Insights from Prof. Dr. Wolfgang Kunz
Sarcomas are a rare and heterogeneous group of malignant tumors that pose significant challenges in both diagnosis and treatment. Within the framework…

Standardized Reporting Template for Sarcomas in the BZKF BORN-Project: Interview with Prof. Dr. Wolfgang Kunz of LMU Munich
Sarcomas are a rare and highly diverse group of malignant tumors that affect both soft tissue and bones, posing significant challenges due to their…

RACOON FADEN Project Tackles Early Detection of Adenomyosis
Endometriosis is a vastly under-researched condition affecting women, but it is finally receiving the attention it deserves through the RACOON FADEN…