A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
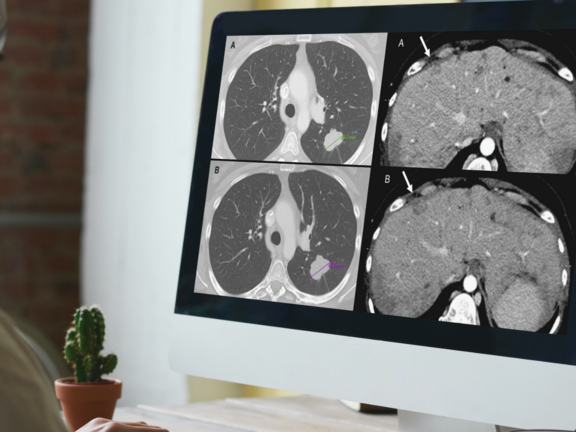
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources

Enhancing flexibility and communication while saving costs and time in clinical trials
Emily Ferris and Alex Arbuckle describe how using mint Lesion™ has enhanced their clinical trial operations, enabling “a more cooperative approach…

University Hospital Jena: Next-level Workflow Optimization in Clinical Trials
How to set up a well-running clinical trial center that can handle up to 40 trials at once has been successfully demonstrated by Laura Graziani, study…
Structured Reporting of Solid and Cystic Pancreatic Lesions in CT and MRI: Consensus-Based Reporting Templates of the German Radiological Society (DRG)
During the daily clinical routine, radiological reports on pancreatic lesions are often written as freeform texts. This causes several problems, in…