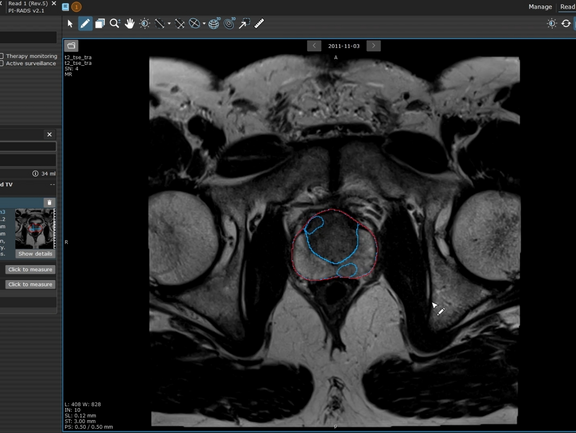
The Prostate.Carcinoma.ai plug-in, developed by our partner FUSE-AI, is a powerful addition to mint Lesion, designed specifically to enhance prostate cancer screenings. This advanced solution leverages artificial intelligence to automate and accelerate prostate MR evaluations based on the PI-RADS 2.1 guidelines, transforming the workflow and precision for radiologists.
At the click of a button, Prostate.Carcinoma.ai performs full segmentation of the prostate and its anatomical zones, and detects and segments potentially malignant lesions. mint Lesion maps each finding onto the standardized prostate lesion scheme, eliminating the need for time-consuming manual lesion annotation and drawing.
Radiologists maintain full control over the AI-generated results, with the ability to review, reject, modify, or add new findings to ensure accuracy. Based on these inputs, mint Lesion automatically calculates the PI-RADS score, delivering a comprehensive and structured report.
mint Lesion also enables longitudinal tracking of prostate lesions for MRI-based active surveillance. Radiologists can easily monitor changes in lesions over time, supporting informed decision-making for patient management and enhancing the continuity of care.
Learn more on our partner page.