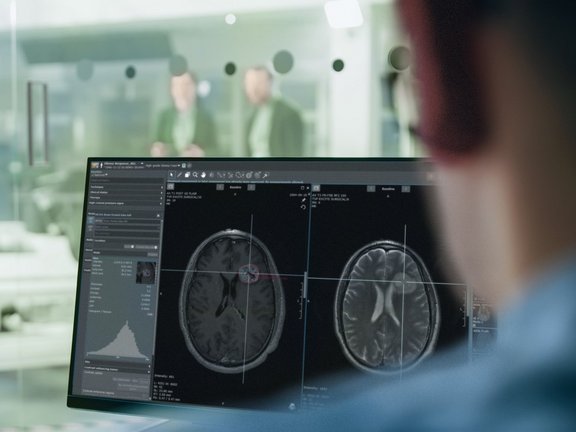
Glioblastomas and other gliomas are the most common malignant primary brain tumor types, yet few effective therapies are available. Clinical research and clinical trials are essential in supporting therapeutic advances in this critical indication. Over a decade ago, the Response Assessment in Neuro-Oncology (RANO) criteria emerged to bolster the consistency of clinical trial findings and facilitate cross-trial comparisons. The recent publication of RANO 2.0[1] is an important update that provides one single set of criteria for investigators to use for clinical research in glioma. It aims to eliminate ambiguity in trial criteria selection, enhance the consistency and comparison of trial data, and potentially expedite the creation of more effective therapies for patients with brain tumors.
In recent years, Mint Medical has concentrated on neuro-oncology for both adult and pediatric primary brain tumors. With a suite of neuro-oncology response criteria, which includes the pediatric guidelines RAPNO for High-Grade Glioma, Low-Grade Glioma, and DIPG, as well as RANO (1.0) for adult High-Grade Glioma, adult RANO Low-Grade Glioma, and RANO - Brain Metastases, we plan to integrate RANO 2.0 into mint Lesion™ in the near future.
Notably, while two-dimensional product of the perpendicular (PPD) measurements remain the standard for this criteria, mint Lesion™ enables both PPD and volumetric evaluations to be conducted concurrently. The forthcoming RANO 2.0 read template in mint Lesion™ will support a configuration to utilize PPD and/or Volume from the same measurements and will derive an overall response for both PPD and Volumes based on the guidelines provided in RANO 2.0. This capability and feature specific to mint Lesion™ will further support the ongoing validation of volumetrics in clinical trials for primary brain tumors.
[1] Wen PY, , et al, RANO 2.0: Update to the Response Assessment in Neuro-Oncology Criteria for High- and Low-Grade Gliomas in Adults. J Clin Oncol. 2023 Sep 29:JCO2301059. doi: 10.1200/JCO.23.01059. Epub ahead of print. PMID: 37774317.