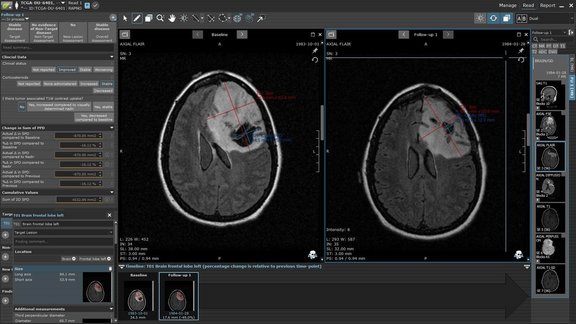
Dr. Anthony Tolcher, medical oncologist and CEO of NEXT Oncology, spoke to us about objectivity as one of the biggest challenges in clinical trials and how a software solution for tumor assessment can improve the quality and reproducibility of data, all while saving time.
Moreover, such software solutions are a key aspect for the approval of novel drugs based on small sample size – like for example targeted or tissue agnostic medicine, as a significant advance for precision therapies. Dr. Tolcher describes how mint Lesion™ assists in generating trustworthy data, “so that [one] can ensure that the drugs that are getting approved really do work.”
Click here or on the image above to watch the full video on YouTube.