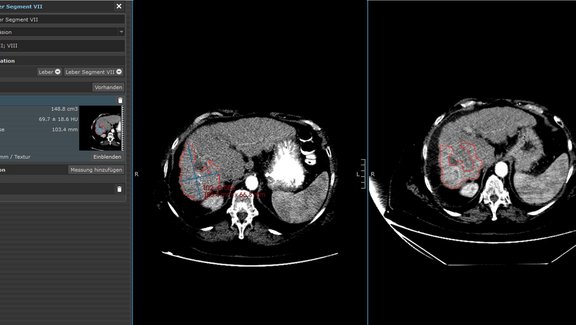
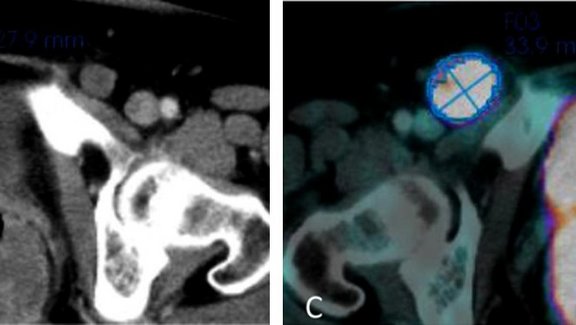
Dr. Laura Oleaga from Hospital Clinìc de Barcelona explains how she and her team have benefitted from implementing mint Lesion™ in the face of an increasing volume of clinical trials. She highlights how standardized reading procedures and reports limit the possibility of errors in clinical trial work, improve the communication between radiology and oncology, and deliver the necessary documentation for trial audits.
Moreover, she decsribes the potential that arises by using mint Lesion™ in their clinical routine – extending clinical trial excellence to the routine care of each oncological patient.
Click here or on the image above to watch the full video on YouTube.