A recent study[1] conducted by researchers at the University Hospital Munich (LMU) investigated the role of tumor growth rate (TGR) in predicting the efficacy of chimeric antigen receptor T-cell therapy (CAR-T) in patients with relapsed or refractory non-Hodgkin's lymphoma. The study explores the association between TGR dynamics from pre-treatment to post-treatment imaging and the patients' outcomes.
The researchers conducted a single-center study involving 59 patients with various lymphoma subtypes who underwent CD19-specific CAR-T therapy between January 2019 and June 2022. The study utilized imaging data, including computed tomography (CT) and 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT, to assess tumor burden and TGR at different time points.
mint Lesion™ was used to conduct all imaging analyses, including the measurement of the sum of the product of diameters (SPD) of up to six target lesions (TL) based on Lugano classification at pre-baseline (pre-BL), Baseline (BL), and first Follow-Up (FU1) imaging to determine tumor burden (TB). For 18F-FDG PET/CT imaging, the Deauville score was calculated to assess metabolic response, with a score of 1–3 indicating a complete metabolic response according to Lugano criteria. Additionally, the software facilitated the measurement of spleen size, with splenomegaly defined by a vertical length exceeding 13.0 cm. Response evaluation at 30-day FU1 CT was based on Lugano criteria, classifying responses as complete response (8 patients, 13.6%), partial response (25 patients, 42.4%), stable disease (15 patients, 25,4%), and progressive disease (11 patients, 18.6%).
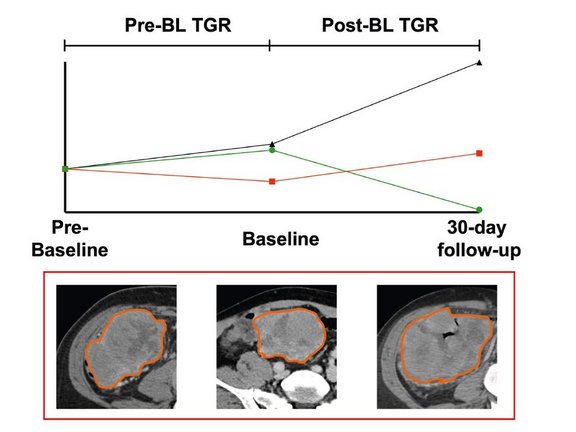
Patients with progressive disease (PD) were divided into two subgroups based on changes in tumor growth rate (TGR) from pre-BL to post-BL.
Among the 11 PD patients, 7 (64%) showed an increase in TGR (PD TGRpre-to-post-BL INCR), while 4 (36%) exhibited a decrease in TGR (PD TGRpre-to-post-BL DECR). Those in the PD TGRpre-to-post-BL DECR group had a comparable overall survival (OS) to patients classified as having stable disease (SD). In contrast, patients in the PD TGRpre-to-post-BL INCR group demonstrated significantly shorter OS (65 days vs 471 days, p<0.001).
The main findings highlight the significance of TGR dynamics in predicting patient outcomes, especially in those classified as having progressive disease (PD) by conventional Lugano criteria. The study suggests that the modification of response criteria to include TGR could enhance the early prediction of patient response and survival.
This research provides valuable insights into the potential of TGR as a novel prognostic imaging biomarker in the context of CAR-T therapy for patients with relapsed or refractory non-Hodgkin's lymphoma. However, the study acknowledges its limitations, such as its single-center nature and the relatively small sample size, emphasizing the need for larger prospective studies to validate the findings. The results encourage further investigation into the utility of TGR as an early response biomarker and its potential benefits in predicting patient outcomes.
[1] Winkelmann M, Blumenberg V, Rejeski K, et al Modification of Lugano criteria by pre-infusion tumor kinetics improves early survival prediction for patients with lymphoma under chimeric antigen receptor T-cell therapy. Journal for ImmunoTherapy of Cancer 2023