A recent prospective study [1] conducted by researchers at Cincinnati Children’s Hospital Medical Center compared manual 2D, semi-automated 2D, and volumetric measurement strategies for tumor response assessment in diffuse intrinsic pontine gliomas (DIPG).
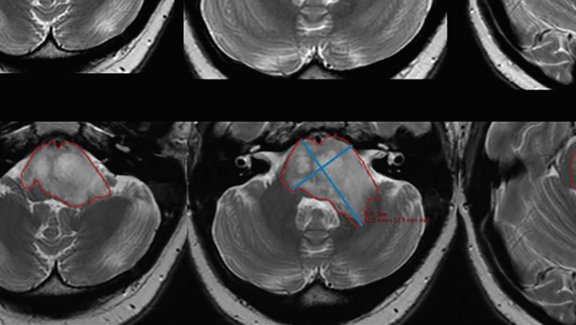
Imaging-related tumor response criteria used to assess DIPGs are based on a longitudinal comparison of two perpendicular measurements made on the axial plane with the largest area of the tumor. “However, these standard tumor measurements were not developed and may not be best-suited for DIPG, and there remains an overall lack of standardization in DIPG measurements, “the authors explained. “2D measurements of diffuse intrinsic pontine gliomas are limited by variability, and volumetric response criteria are poorly defined.”
As part of Phase I/II study of Ribociclib (Novartis, ClinicalTrials.gov Identifier: NCT02607124), ten patients were prospectively recruited to undergo MR imaging before and following drug therapy. Clinical 2D cross-product values were calculated by multiplying manually measured linear long and short axis, and mint LesionTM was used to derive maximum cross-product and tumor volume automatically.
The comparison of the three measurement strategies showed the weakest correlation of change in tumor size between manual 2D and semi-automated 2D measurements (r = 0.36, P = .011). “Manual 2D cross-product measurements may underestimate tumor size and disease progression compared with semi-automated 2D and volumetric measurements,” the researchers determined. This discrepancy is presumed to origin in the variable morphology of DIPGs, but also in the radiologists’ choice of section location and measurement orientation, which is often similar to the ones in previous examinations. The latter bias is supported by the study’s results, with “important implications for the use of segmentation software in clinical practice.”
Future studies are needed to identify the relationship between true volumetric and linear measurements of tumors with non-spheric growth patterns to allow comparison between studies with and without 3D measurements. Another area of further investigation could be examining correlations between tumor measurements and/or response assessment and clinical outcomes. “Application of semi-automated 2D and volumetric measurements in therapeutic trials will alter response assessment compared with standard 2D measures in DIPG, “the authors concluded. “Further research is needed to outline relationships among these methods, clinical signs of progression, and survival.”
[1] L.A. Gilligan, M.D. DeWire-Schottmiller, M. Fouladi, P. DeBlank and J.L. Leach Tumor Response Assessment in Diffuse Intrinsic Pontine Glioma: Comparison of Semiautomated Volumetric, Semiautomated Linear, and Manual Linear Tumor Measurement Strategies
American Journal of Neuroradiology May 2020, 41 (5) 866-873, https://doi.org/10.3174/ajnr.A6555