A study conducted by researchers at University Hospital Tuebingen retrospectively compared two liver-targeted therapies for uveal melanoma patients with liver metastases. The results showed that Chemosaturation-Percutaneous Hepatic Perfusion (CS-PHP) had significantly longer overall survival (516 days) compared to Transarterial Radioembolization (SIRT) (300.5 days).
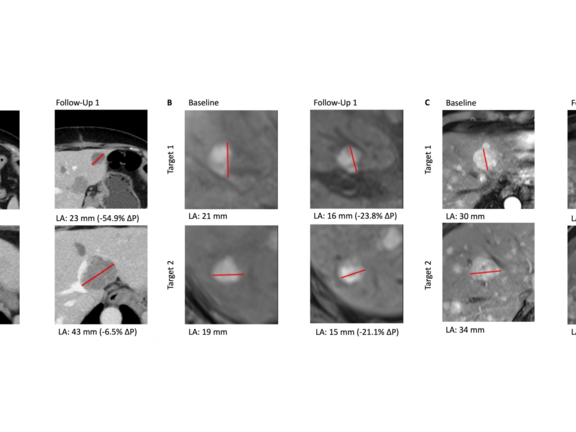
mint Lesion™ was used to assess the treatment efficacy in line with the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). The study observed that the disease control rate, which includes Complete Response (CR), Partial Response (PR), and Stable Disease (SD), was 18% for SIRT and 30% for CS-PHP.
These findings highlight the need for further research and prospective randomized trials to optimize liver-targeted treatment strategies and address the challenges faced by patients with this condition. Learn more.