A recent retrospective study led by Dr. Marilyn J. Siegel and her team at the Washington University School of Medicine in St. Louis has shed light on a critical issue in cancer care: routine clinical reads are more prone to overdiagnosing progressive disease when compared to RECIST 1.1 interpretations. This discrepancy holds significant implications, potentially leading to the premature discontinuation of effective treatments for cancer clinical trial participants and patients under standard care.
In this study, mint Lesion software was utilized for the criteria-based reads, determining overall response assessments according to RECIST 1.1 criteria, and generating structured reports for the clinical trial's principal investigator.
To learn more about the study's insights into the discrepant assessments and the suggested steps for mitigating this issue, click here.
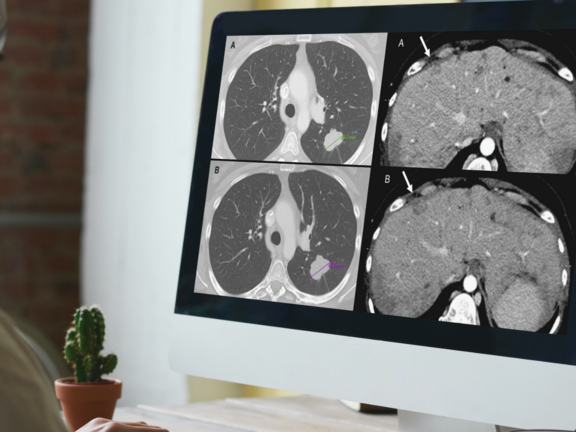
Study Discovers Overdiagnosis of Progressive Cancer in Routine Clinical Evaluations
Related Resources
Related Resources
RACOON – Imaging, Data & Collaboration for Better Decisions
Modern radiology faces a central question: how can imaging and clinical data be combined in a way that leads to more precise diagnoses,…

Rethinking Early Detection: How RACOON-MARDER Aims to Spot Liver Cancer Sooner
Hepatocellular carcinoma (HCC) is often diagnosed too late, limiting treatment options and survival. The RACOON-MARDER project aims to change that. By…

mint Lesion Research Suite: The Flexible Imaging Research Platform Built for Scalable, Single- and Multi-Center Studies
mint Lesion's Research Suite is an advanced imaging research platform designed to support academic teams in streamlining and scaling their imaging…