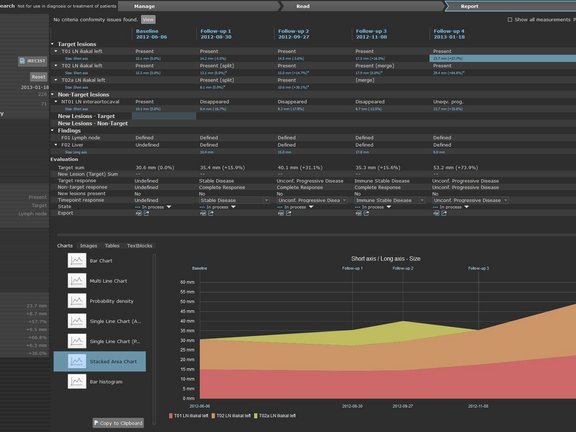
Shortly following the release of the new iRECIST- oncologic response criteria (Lancet Oncology, March 2017) the University Medical Center in Tübingen, Germany had already begun to implement this new response criteria into their clinical routine using mint LesionTM. The iRECIST guidelines are intended to provide a standard approach to the evaluation of solid tumors with measurements and assessment of the disease burden in trials where an immunotherapy is used.
PD Dr. Bernhard Klumpp who provides radiological response evaluation of of studies is one of the first radiologists to apply the new iRECIST response assessment in mint LesionTM. “The possibility of transferring the existing report process to an iRECIST process is of course sensational. For this a new measurement is not necessary, one simply changes perspective during observation of the process. Moreover, special features of iRECIST are indicated in the application”, says Dr. Klumpp.
The new iRECIST response evaluation platform is now available for all mint LesionTM Users. iRECIST is expected to be widely applied in both routine radiology evaluation as well as for application as a standardized response assessment methodology as required in oncology clinical trials.