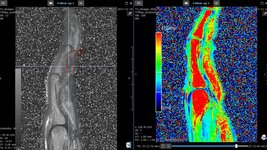
University of Miami & Nova Souteastern University: Study shows feasibility of radiomics and T2 mapping as novel imaging biomarkers of activity in superficial fibromatosis
A retrospective study [1] conducted by researchers at University of Miami Miller School of Medicine and Nova Southeastern University investigated whether quantifying the heterogeneity of the MR…
German Radiology Congress 2021
https://www.roentgenkongress-industrieportal.de/partner/mint-medical/
The benefits of structured oncologic reporting with mint Lesion™
Dr. Damiano Caruso of Sapienza Universita di Roma recounts how he got to know mint Lesion™ at a workshop hosted by the ESOI. Among other workstations, mint Lesion™ automatically stood out by being…
mint Lesion™ 3.8. Release: What’s New?
The recent 3.8 release brings with it a multitude of new features and refinements that further facilitate reporting, interdisciplinary collaboration, and patient management in clinical routine and…
SLK Clinics Heilbronn: Oncological Therapy Assessment and Efficient, Structured Reports in Daily Routine
As a clinic group with almost 1500 beds, the SLK Clinics Heilbronn are the largest healthcare provider in the Heilbronn-Franconia region. The clinic for “Diagnostic Radiology, Minimally Invasive…
Project „KoMed“: The cognitive medical assistant for risk and complication prevention
Funded by the Ministry of Science, Research and the Arts of Baden-Wuerttemberg, Mint Medical has been supporting the Heidelberg University Hospital as a cooperation partner in an innovative project…
Structured reports for better patient care in oncology
“mint Lesion™ enables us to make better reports because it truly provides all the necessary data for patient care.” Dr. Laurent Chapuis shares how the software solution mint Lesion™ makes the…
Workflow optimization, increased efficiency and reduced errors in clinical trials
"It's a paradigm shift." This is how Prof. Ulf Teichgräber, Director of the Institute of Diagnostic and Interventional Radiology at the University Hospital Jena, describes the improvement in…
Mint Medical is now part of Brainlab AG, Munich
We are very happy to be part of the Brainlab family. We are convinced that together we will make a huge impact on the future of healthcare – and this future has already begun! What does this…