Optimizing Glioblastoma Imaging: Enhancing MRI Efficiency and Quality with Deep Learning
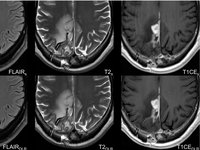
This study investigates the use of deep learning (DL) to optimize MRI protocols for glioblastoma patients, aiming to reduce scan time and improve image quality. Glioblastomas are aggressive brain…
University Hospital Tübingen: Advancing MRI Efficiency in Glioblastoma Care with Deep Learning
This study explores the use of deep learning (DL) to optimize MRI protocols for glioblastoma patients. Glioblastomas, known for being the most prevalent and most aggressive malignant brain tumors in…
Insights into the BORN Project: Development and Successes with Dr. Maurice Heimer
The BORN-project of the Bavarian Center for Cancer Research (BZKF) is making swift progress in its second funding phase. A key aspect of the project is the development of structured reporting…
A Closer Look at the BZKF BORN-Project: Interview with Dr. Maurice Heimer from the Clinic and Polyclinic for Radiology at the LMU Klinikum
The Bavaria-wide Oncological Radiology Network (BORN) is now in its second funding phase and is making rapid progress. The project was initiated in August 2022 with the goal of improving cancer…
Key Imaging Features for Differentiating Rare Pancreatic Tumors
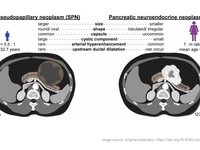
Solid pseudopapillary neoplasms (SPN), also known as Frantz tumors, are rare tumors of the pancreas. Due to overlapping features of SPN and pancreatic neuroendocrine neoplasms (pNEN), distinguishing…
Heidelberg University Hospital: Key Imaging Features to Distinguish Solid Pseudopapillary Neoplasms from Pancreatic Neuroendocrine Neoplasms
Solid pseudopapillary neoplasms (SPNs), or Frantz tumors, are rare pancreatic tumors accounting for 2-3% of all pancreatic neoplasms. These tumors typically exhibit low malignant potential.…
2,237 Patients, 11 Hospitals, four HCC Criteria: A Comparison Study
A recent study, conducted across 11 South Korean hospitals, compared the diagnostic performance of four hepatocellular carcinoma (HCC) diagnostic guidelines and readers' judgments. The study was…
Multicentric Study: Comparison of Diagnostic Guidelines for Hepatocellular Carcinoma
Recent advancements in MRI techniques and tumor biology have led to updated hepatocellular carcinoma (HCC) diagnostic guidelines from various liver study associations. Conducted across 11 South…