Mint Medical is on the front lines in the fight against COVID-19, proving that radiology can act as a role model for gathering evidence by utilizing structured data.
In this webcast, Prof. Dr. Maria-Katharina Ganten and Gabriel Salg highlight the urgent need for structured data collection, as well as the importance of data quality, integrity, and traceability. The hosts discuss how to support trial compatible data acquisition in routine work by example of a European multicenter project.
Prof. Ganten and Mr. Salg also provide a look to the future in which radiology has the opportunity to set standards for new research strategies, not only concerning COVID studies but rather for any disease or treatment.
Clinical trial excellence for routine covid-19 assessment
Related Resources
Related Resources
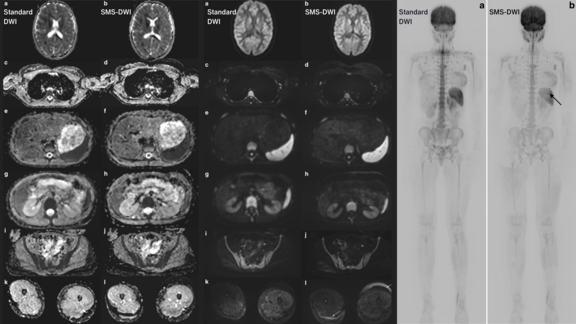
University Hospital Jena: Research Investigating the Viability of Accelerating Whole-Body MRI in Children and Adolescents through STIR DWI with Simultaneous Multi-Slice Excitation
This study[1] addressed the challenges of conducting WB-MRI in paediatric patients, particularly the prolonged acquisition time required for…

RACOON FHIR Workshop: Empowering Healthcare Research via Enhanced Interoperability
This week Brainlab hosted the RACOON FHIR Workshop with around 40 participants. The workshop was organized by Mint Medical and supported by Snke OS,…
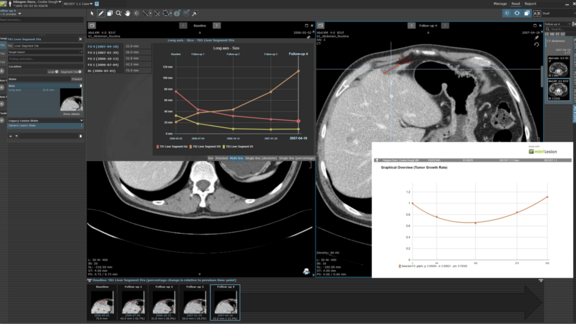
Tumor Growth Rate Modeling: A Novel Approach to Evaluating the Efficacy of Cancer Therapies
The 2020 review of Clinical Trial Evidence Supporting US Food and Drug Administrative Approval of Novel Cancer Therapies Between 2000 and 2016…
