Mint Medical is on the front lines in the fight against COVID-19, proving that radiology can act as a role model for gathering evidence by utilizing structured data.
In this webcast, Prof. Dr. Maria-Katharina Ganten and Gabriel Salg highlight the urgent need for structured data collection, as well as the importance of data quality, integrity, and traceability. The hosts discuss how to support trial compatible data acquisition in routine work by example of a European multicenter project.
Prof. Ganten and Mr. Salg also provide a look to the future in which radiology has the opportunity to set standards for new research strategies, not only concerning COVID studies but rather for any disease or treatment.
Clinical trial excellence for routine covid-19 assessment
Related Resources
Related Resources

Customizable reading templates for therapy response assessment
mint Lesion offers customizable reading templates for therapy response assessment in clinical care and research. Adapt parameters, define lesion…

RACOON-RESCUE is Advancing Pediatric Non-Hodgkin Lymphoma Care
Pediatric Non-Hodgkin Lymphoma (NHL) is the fourth most common tumor in children and adolescents, yet its radiological methods lack standardization,…
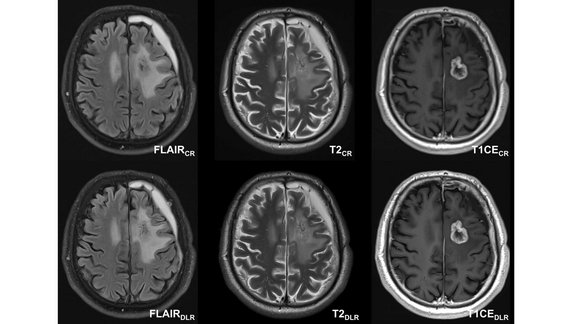
University Hospital Tübingen: Neuro-Oncological Imaging with Deep Learning Reconstruction (DLR)
A recent study conducted by the University Hospital of Tübingen investigated the potential of deep learning reconstruction (DLR) in magnetic resonance…
