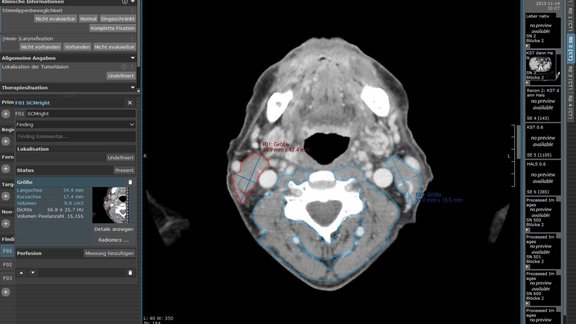
The Bavaria-wide Oncological Radiology Network (BORN) is now in its second funding phase and is making rapid progress.
The project was initiated in August 2022 with the goal of improving cancer care in Bavaria through standardized imaging and reporting. Within the network of the Bavarian Center for Cancer Research (BZKF), a unified research environment is being created to benefit both patients and medical professionals across Bavaria.
Through the collaboration of the six Bavarian university hospitals in Augsburg, Erlangen, the two locations in Munich, Regensburg, and Würzburg, knowledge and expertise in the areas of early detection, therapy, and follow-up care for tumors are pooled. This enables comprehensive and interdisciplinary care for patients.
A key aspect of the project is the development and uniform use of structured reporting templates by the participating university hospitals. These templates are systematically created and shared to ensure the efficiency and consistency of radiological diagnoses.
We spoke with Dr. Maurice Heimer from the Clinic and Polyclinic for Radiology at the LMU Klinikum in Munich about the challenges and opportunities in developing reporting templates. We reflect on the first phase of the project, in which the LMU Klinikum was responsible for developing the structured reporting template for lung cancer.
Dr. Heimer, what is it like for you and LMU to be part of this major standardization project?
Together with Dr. Spiro, I co-moderate the working group for lung cancer. Our collaboration with dedicated colleagues from across Bavaria has allowed us to make significant progress in harmonizing protocols and developing a software-based, structured reporting template. We view what we've achieved together so far as a success and look forward to the second funding phase!
For us, it is crucial to actively shape BORN and use it as a platform for intensive, cross-regional collaboration. Besides our work on lung cancer, we are also involved in a larger team working on developing other reporting templates for different tumor entities. This allows us to bring our expertise and commitment to quality and precision into the structured reporting templates, which aim to significantly improve the quality of reporting in clinical routine. Additionally, structured reporting also offers added value for scientific studies in oncological imaging, especially when multiple centers are involved.
What challenges have you encountered in defining the reporting template? And how were they addressed?
All locations were interested in structured reporting for lung cancer, but their initial visions were still different. A major challenge was developing a common reporting template that is clinically pragmatic and captures all essential oncological findings in a structured manner. The breadth and depth of the structured reporting templates were actively discussed in many meetings with the involved experts to precisely capture all relevant parameters for evidence-based therapy management while leaving enough flexibility for important findings that are not primarily associated with the tumor disease.
How does the LMU proceed within the BORN project?
Now that protocols and reporting structures have been harmonized at all locations, the agreed-upon reporting templates can be used in routine clinical reporting. The software needs to be rolled out at all locations and integrated into the local IT infrastructure. The goal of the current phase is to clinically establish the reporting template at all BORN sites, continuously improve the structure of the template, and gather feedback from referrers and colleagues. Looking ahead, we plan to leverage the experiences and prospectively collected data from our collaborative work on structured reporting for scientific multicenter projects.
How do you reflect on your collaboration with Mint Medical and the training sessions?
The collaboration with the developers was highly committed and productive, both in structuring the reporting templates and in their on-site clinical implementation. During regular development meetings with radiological expert groups, the developers successfully integrated the radiological reporting structure and clinically relevant staging categorizations into the template. Looking ahead, the structured reporting developed within the BORN project has the potential to significantly improve the quality and completeness of the longitudinal documentation of oncological findings. We are contributing to this effort together with Mint Medical.
Dr. Maurice Heimer is a radiology resident at the Clinic and Polyclinic for Radiology at the LMU Klinikum Munich. He is also a co-opted board member of the AG Oncological Imaging in the German Radiological Society and a member of the young committee of the European Society of Oncologic Imaging (ESOI).