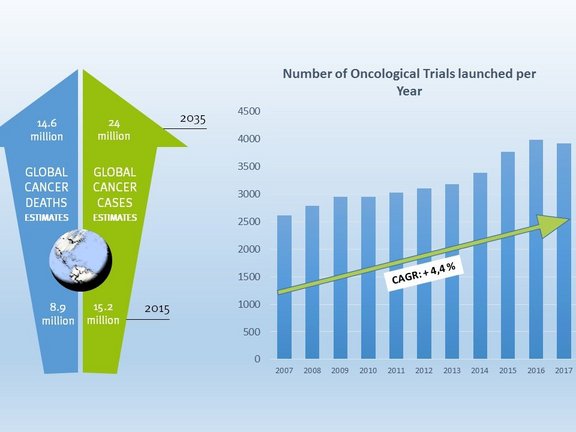
Cancer cases and cancer related deaths continue to increase globally. While new cancer therapies are being identified offering new options for patients, many patients continue to live with cancer as a chronic disease. Nevertheless, many still succumb to their disease. In 2015, there were nearly 9 million cancer related deaths globally; by 2035 this number is anticipated to reach more than 14 million. Over the same period, the number of cancer cases is expected to increase from 15 million cases to 24 million. (AACR Cancer Progress Report 2016, page 13)
Clinical trials are a major step in bringing new treatments for these patients. They also offer new ways to detect, diagnose, and reduce the risk of cancer. Clinical trials are not intended as a means to treat the patient but rather to investigate the therapeutic. In many cases however, they are a patient’s last option.
As cancer is a huge and worldwide challenge it is of little surprise that of all clinical trials, oncological trials are the greatest in number. Since 2007 more than 35,000 oncological trials have been launched. This amounts to 17.4% of all trials conducted within the last ten years. Over the past ten years, the number of oncological trials being initiated per year has grown by 60% from 2,500 in 2007 to nearly 4,000 in 2017. This translates into a compound annual growth rate (CAGR) of 4.4% testifying to the increasing importance of oncological research. Almost 50% of oncological trials are conducted in the United States; a fact that highlights the country’s outstanding role in cancer research. (clinicaltrials.gov)