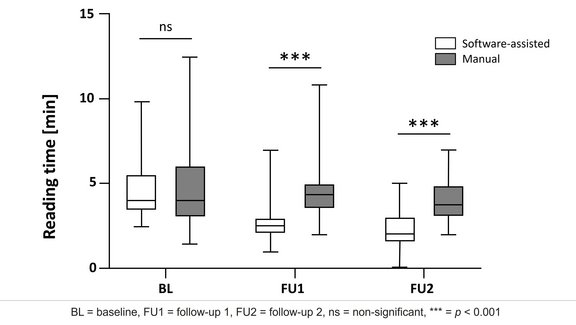
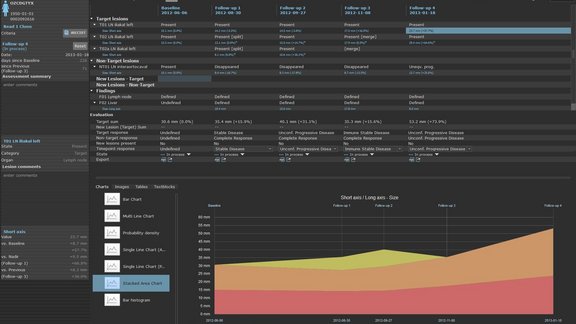
We are happy to present our newly published whitepaper on iRECIST and its application. This is available here on our homepage as well as on www.irecist.com. Mint Medical had already implemented the iRECIST criteria in May 2017 for its new version of the mint LesionTM software.
RECIST has been the standard for therapy response assessment in solid tumors in clinical trials for several years. It provides a reproducible methodology for the assessment of clinical and imaging data as well as the evaluation of therapy response. This evaluation is based on the development of tumor burden through various timepoints. When it comes to the evaluation of immuno-therapies, however, RECIST in its existing form may not always be able to represent the therapy response appropriately. Therefore, iRECIST has been developed. Unlike RECIST, iRECIST also takes into account specific immuno-therapy parameters. For instance, it considers the possibility of a so-called pseudo-progression where a tumor would grow or new lesions would appear before showing a response to the treatment.
With the integration of iRECIST into mint LesionTM, we offer the possibility to use both RECIST and iRECIST for therapy response assessment. Sites in Europe, Asia and the USA have already begun using the new criteria in the evaluation of clinical trials.