In the first week of March, we will make the relevant software functions available for all our current users at no cost. We plan to offer the COVID-19 reading template also to institutions with no on-premise installation of mint LesionTM. We will then invite hospitals and radiological practices to perform appropriate diagnostic procedures using a cloud-hosted version of mint LesionTM, where they can upload and assess de-identified data in compliance with GDPR requirements.
We want to encourage radiologists to contribute to the availability of primary data that can be analyzed scientifically. Hence helping to improve care by evidence resulting from high-quality primary data in a growing database.
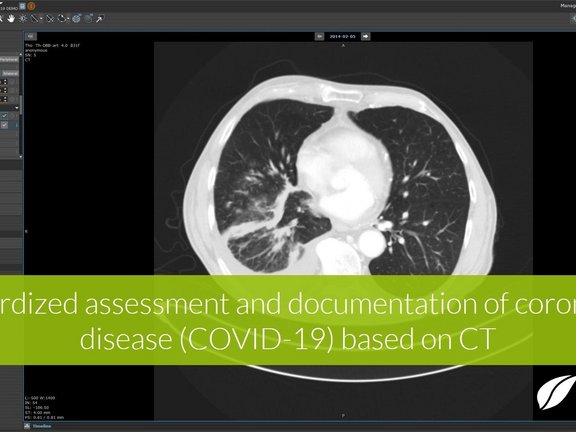
Recent research shows that CT imaging must be considered as a primary analysis tool for the diagnosis of the infection, the monitoring of disease progression, and the assessment of the efficacy of therapies and vaccine candidates. However, this is only possible if the imaging findings are quantitatively collected and evaluated directly on the image on a supra-regional, preferably global level using uniform, comparable criteria. This provides structured data that can be universally shared and analyzed. To support this, we developed a new evidence-based reading template in mint LesionTM to identify and quantitatively characterize the typical features of viral pneumonia. The template supports the measures for the detection and systematic statistical recording of the course of COVID-19 infections as well as research into therapies and vaccine candidates. In addition to radiological parameters, the template supports the documentation of related clinical information. All data is collected under consideration of research regulations, including audit trails, to ensure data quality, integrity, and traceability local as well as multi-center cohort investigator-initiated trials.
mint LesionTM provides computer-assisted and context-guided data capture and annotations in easy-to-use software. The particular strength of mint LesionTM lies in the fact that any measured value is immediately linked to its context and further related data of clinical significance. Hence, the data is integrated into a digital model of the patient and its evolution over time. Mint Medical abides by the tenet of no data being left behind on the image. mint LesionTM generates minable data in a semantic data model with a HIPAA-and FDA CFR Part 11-ready audit trail for Phase I-III clinical trials and medical research.
In the mint LesionTM software, we assist the users with an overview of the current literature related to CT-based diagnosis of the COVID-19 disease. Furthermore, we provide extensive documentation about how the template has been created and which evidence has been used to define the data elements. In the case of new publications, we will update the template in a way that preserves already collected data.
Data gathering and exchange for speeding up Imaging Biomarker Research
With the goal of a supra-regional data collection and evaluation, Mint Medical aims to contribute to the establishment of a central database to be coordinated by a trustworthy body, like for instance, a public operator, university hospital, research group, or contract research organization. In this database, de-identified data obtained by participating institutions can be aggregated according to reproducible criteria. The aggregated data can serve as a high-quality source for researchers working on the development of COVID-19 diagnostics, therapies, and vaccines. Additional related research and analysis up to the training of AI-algorithms have already started.
All data collected using the COVID-19 read template can be analyzed with the mint AnalyticsTM software add-on. Furthermore, it is exportable in various human- and machine-readable data formats for external analysis. All image annotations can be exchanged in open formats, like NRRD, or as DICOM-compliant annotations (DICOM RT, DICOM Seg. Surface Obj.). Interfacing with other information systems, such as hospital information systems to include clinical data, is possible through standardized interfaces, including HL7, FHIR, and openEHR.
As part of our contribution to speeding up research, we also provide participating institutions with a draft for an application form for the ethics board approval.
Overview of recent research studies on the feasibility of CT imaging and its advantages over the RT-PCR method
At present, a "real-time polymerase chain reaction" (RT-PCR) method is used to detect the presence in the blood of nucleic acid chains characteristic of the virus. However, this method does not provide quantitative data on the stage of the disease. A new study [1] alarmingly found that the sensitivity of RT-PCR at (+3/-3) days after the appearance of external symptoms is only 71%, i.e. the first RT-PCR test fails to detect the infection in almost 1/3 of patients with relevant symptoms. The study also found that in 98% of patients at the same stage, abnormalities that are characteristic of viral pneumonia are visible in computed tomography (CT) of the lungs.
In another study [2], researchers from China and the Netherlands examined data from 1,014 patients and concluded that “CT imaging has high sensitivity for diagnosis of COVID-19. Our data and analysis suggest that chest CT should be considered for the COVID-19 screening, comprehensive evaluation, and following-up”. One other study [3] concludes that there are correlations between the stage of the disease and the radiological appearance: “Recognizing imaging patterns based on infection time course is paramount for not only understanding the disease process and natural history of COVID-19 but also for helping to predict patient progression and potential complication development”.
"Chest CT examination plays an important role in the initial diagnosis of the novel coronavirus pneumonia" [4] and "CT plays an important role in the diagnosis and evaluation of this emerging global health emergency," [5] state the authors of two other very recent publications.
Further related information
"We’ll need diplomatic efforts to drive international collaboration and data sharing. Developing antivirals and vaccines involves massive clinical trials and licensing agreements that would cross national borders. We should make the most of global forums that can help achieve consensus on research priorities and trial protocols so that promising vaccine and antiviral candidates can move quickly through this process.", so Bill Gates in his blog post on How to respond to COVID-19. And prepare for the next epidemic, too. "The goal of this work should be to get conclusive clinical trial results and regulatory approval in three months or less, without compromising patients’ safety."
In a joint statement, the German Research Foundation and the National Natural Science Foundation of China encourage the scientific community to rapidly respond to the current challenges of COVID-19. Both societies call the scientific community to work together to "provide a scientific basis for strategic analysis and decisions" to strengthen international research collaborations and share their results.
Further current literature on COVID-19 in the context of radiology can be found on the websites of the German Radiology Society and RSNA.