Heidelberg, GER – Mint Medical GmbH, a global MedTech company specialized in software solutions for data-driven radiology, today announced that it provides a new reading template for the quantitative diagnosis of abnormalities that are characteristic of the coronavirus disease 2019 (COVID-19). This template aims to standardize the assessment and data collection of COVID-19 disease. Hence, Mint Medical establishes the infrastructure for data gathering in the course of future single- and multi-center cohort studies to generate minable data and gain insights into the characteristics of COVID-19. “We want to encourage radiologists to contribute to the availability of primary data that can be analyzed scientifically. Hence helping to improve care by evidence resulting from high-quality primary data in a growing database,” says Dr. Matthias Baumhauer, Managing Partner at Mint Medical.
In the first week of March, Mint Medical will make the relevant software functions available for all its current users at no cost. The company plans to offer the COVID-19 reading template also to institutions with no on-premise installation of mint Lesion™. The company will then invite hospitals and radiological practices to perform appropriate diagnostic procedures using a cloud-hosted version of mint Lesion™, where they can upload and assess de-identified data in compliance with GDPR requirements.
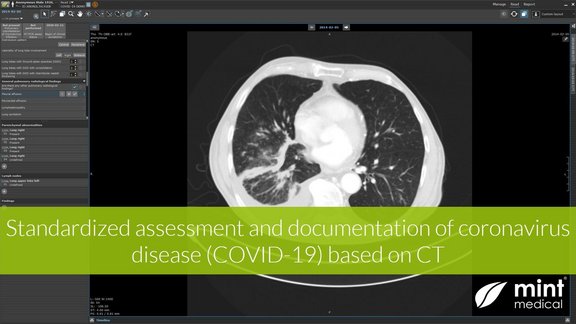
Recent research shows that CT imaging must be considered as a primary analysis tool for the diagnosis of the infection, the monitoring of disease progression, and the assessment of the efficacy of therapies and vaccine candidates. However, “this is only possible if the imaging findings are quantitatively collected and evaluated directly on the image on a supra-regional, preferably global level using uniform, comparable criteria. This provides structured data that can be universally shared and analyzed,” so Dr. Baumhauer. To support this, Mint Medical developed a new evidence-based reading template in mint Lesion™ to identify and quantitatively characterize the typical features of viral pneumonia. The template supports the measures for the detection and systematic statistical recording of the course of COVID-19 infections as well as research into therapies and vaccine candidates. In addition to radiological parameters, the template supports the documentation of related clinical information. All data is collected under consideration of research regulations, including audit trails, to ensure data quality, integrity, and traceability local as well as multi-center cohort investigator-initiated trials.
In the actual mint Lesion™ software, Mint Medical assists the users with an overview of the current literature related to CT-based diagnosis of the COVID-19 disease. Furthermore, it provides extensive documentation about how the template has been created and which evidence has been used to define the data elements. In the case of new publications, Mint Medical will update the template in a way that preserves already collected data.
Data gathering and exchange for speeding up Imaging Biomarker Research
With the goal of a supra-regional data collection and evaluation, Mint Medical aims to contribute to the establishment of a central database to be coordinated by a trustworthy body, like for instance, a public operator, university hospital, research group, or contract research organization. In this database, de-identified data obtained by participating institutions can be aggregated according to reproducible criteria. The aggregated data can serve as a high-quality source for researchers working on the development of COVID-19 diagnostics, therapies, and vaccines. Additional related research and analysis up to the training of AI-algorithms have already started.
All data collected using the COVID-19 read template can be analyzed with the mint Analytics software add-on. Furthermore, it is exportable in various human- and machine-readable data formats for external analysis. All image annotations can be exchanged in open formats, like NRRD, or as DICOM-compliant annotations (DICOM RT, DICOM Seg. Surface Obj.). Interfacing with other information systems, such as hospital information systems to include clinical data, is possible through standardized interfaces, including HL7, FHIR, and openEHR.
About Mint Medical
For ten years, Mint Medical GmbH has been developing and marketing mint Lesion™, the technologically leading software solution for a standardized qualitative and quantitative evaluation of imaging data according to defined criteria, workflows, and guidelines in the context of clinical trials as well as clinical routine. Together with the integrated, visual data analytics software mint Analytics, mint Lesion™ is already used at a geographically large proportion of university hospitals and general hospitals in Germany, Switzerland, as well as Austria, Italy, and the US. Furthermore, it is used by many leading contract research organizations worldwide to assess the efficacy of novel treatments in cancer and other types of diseases.
About mint Lesion™
mint Lesion™ provides computer-assisted and context-guided data capture and annotations in easyto-use software. The particular strength of mint Lesion™ lies in the fact that any measured value is immediately linked to its context and further related data of clinical significance. Hence, the data is integrated into a digital model of the patient and its evolution over time. Mint Medical abides by the tenet of no data being left behind on the image. mint Lesion™ generates minable data in a semantic data model with a HIPAA-and FDA CFR Part 11-ready audit trail for Phase I-III clinical trials and medical research.