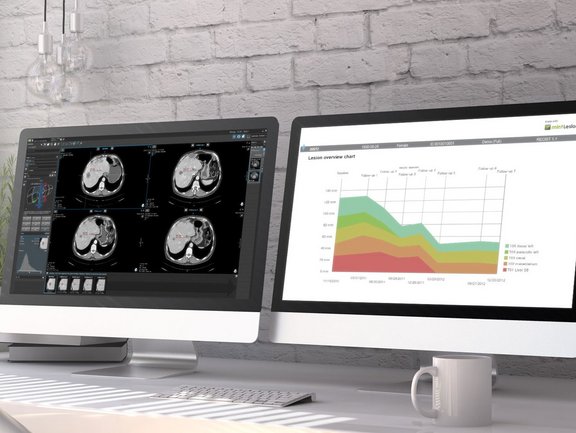
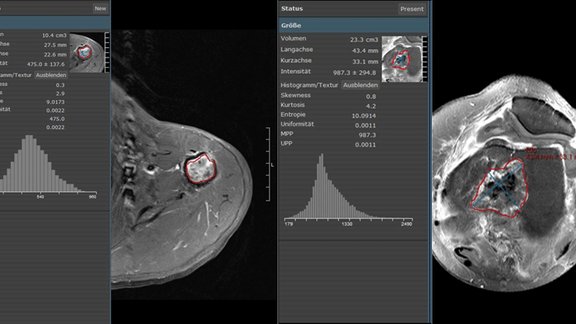
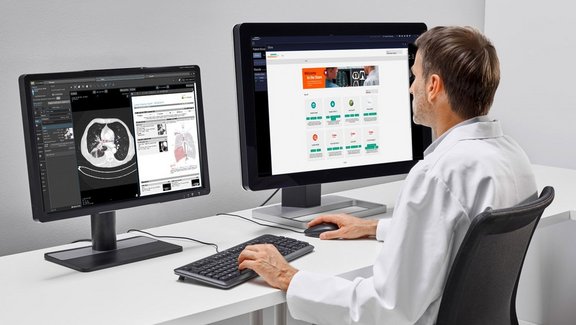
In contrast to clinical trials, unstructured free-text reporting is still common in the clinical routine. Such reports often lack content and clarity, burdening the referring physicians with the time-consuming and cumbersome task of finding and extracting essential information and inferring conclusions rather than immediately comprehending the evidence. In addition, novel cancer therapies often require the use of appropriate response assessment criteria to monitor their effect, which is much easier to accomplish with computer-aided standardized evaluation.
Two recent comparative studies have proven the advantage of computer-aided, criteria-based standardized evaluation over the non-standardized, common practice free-text reporting, using mint Lesion™ as dedicated software.
To discern and quantify the differences between free-text reporting and computer-aided standardized tumor response evaluation, a retrospective study was conducted in 2017, where 100 CTs of patients with cancer eligible for RECIST 1.1 [1] were re-evaluated using mint Lesion™. In pursuit of a similar purpose, another study took place earlier this year, in which CT scans of 50 patients with metastatic renal cell carcinoma were re-evaluated applying iRECIST[2].
Significant differences in tumor response ratings were discovered during both studies. The differences were mainly caused by the rating of even small tumor burden changes as PD or PR, or by comparison to the most recent prior CT scan instead of the comparison to nadir or baseline values.
Both studies thus recommend the use of criteria-based standardized quantitative radiological reporting in routine clinical practice, which fosters data-driven therapy decisions and can be successfully implemented with mint Lesion™.
[1] Goebel, J., Hoischen, J., Gramsch, C. et. al. Tumor Response Assessment: Comparison between unstructured free-text reporting in routine clinical workflow and computer-aided evaluation based on RECIST 1.1 Criteria. J. Cancer Res Clin Oncol (2017).
[2] Schomburg, L., Malouhi, A., Grimm, MO. et al. iRECIST-based versus non-standardized free text reporting of CT scans for monitoring metastatic renal cell carcinoma: a retrospective comparison. J Cancer Res Clin Oncol (2022).