In randomized controlled trials (RCTs), a vast proportion of patients does not meet the precise inclusion criteria. In oncology, for example, two-thirds of the patients are not represented by current RCTs [1]. Real-world data (RWD) studies can provide supplementary information from groups of patients who may not be eligible for RCTs, generating evidence across a broader variety and heterogeneity of cases.
RWD creates new opportunities to complement and enhance the evidence collected in the controlled environment of clinical trials, providing healthcare decision-makers with meaningful information that impacts patient management and regulatory decisions.
However, there are also some limitations associated with real-world data. Not being collected for research purposes, they “are unlikely to appear in an EHR in structured form if at all” [2]. The inconsistent use of terminology as well as differences in formats and content increase data heterogeneity and diminish their quality.
As an example, a recent retrospective observational study [3] evaluated different methods to assess cancer progression from EHR data and concluded that due to missing data and lack of clarity in radiology reports, the RECIST-approach was not feasible. “With a strict RECIST definition (radiologist-defined target lesions required in imaging report), no patient chart (0%) yielded data suitable for assessing cancer progression.” Even with more tolerant criteria, only 31% of the charts directly compared all measured key lesions between two time points.
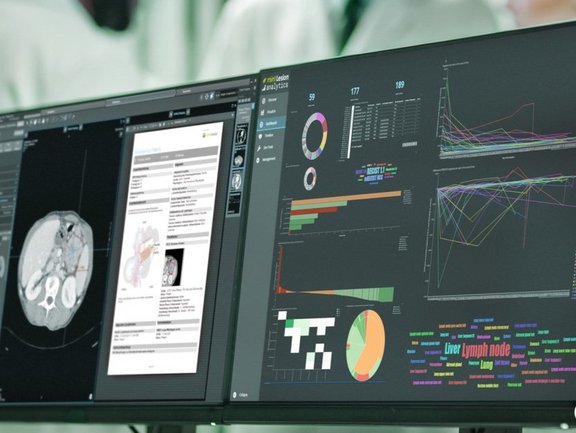
In an interactive and dynamic dialogue with the reader, mint Lesion captures as much data as possible and links every measured value to context data, generating complete and minable reports. In addition, the structured, high-quality annotated data in mint Lesion can be used to augment decision-making in multidisciplinary tumor boards, identify issues as they arise (instead of many months or even years later), or draw up new hypotheses, which go beyond the published literature.
1 Donia,M., Hansen,S. W., and Svane I. M., Real-world evidence to guide healthcare policies in oncology, Oncotarget. 2019 Jul 16; 10(44): 4513–4515
2 Bartlett, V. L., Dhruva, S. S., Shah, N. D. et al. Feasibility of Using Real-World Data to Replicate Clinical Trial Evidence, JAMA Netw Open. 2019;2(10):e1912869
3 Griffith, S.D., Tucker, M., Bowser, B. et al. Generating Real-World Tumor Burden Endpoints from Electronic Health Record Data: Comparison of RECIST, Radiology-Anchored, and Clinician-Anchored Approaches for Abstracting Real-World Progression in Non-Small Cell Lung Cancer. Adv Ther 36, 2122–2136 (2019)