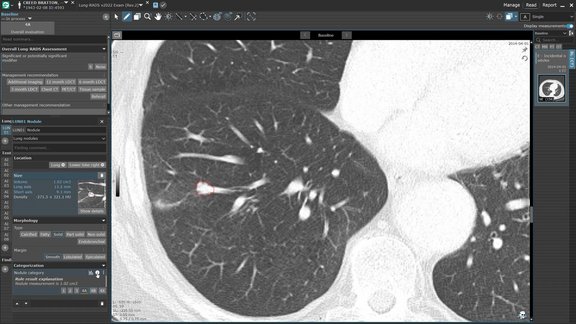
Following the FDA’s recent analysis of the growth rate constant (g) as a prognostic marker1, the challenge for many research organizations is implementation. mint Lesion provides a commercially available software platform that integrates directly into the clinical trial workflow the same mathematical framework for tumor growth rate modeling with g-values cited by the FDA in this important analysis.
The Mathematical Foundation
The FDA analysis utilized a regression-growth model assuming two independent processes: exponential decay (d) and exponential growth (g). mint Lesion implements the four specific models used in this research:
- gd: Biexponential growth and decay
- gx: Growth only
- dx: Decay only
- gdΦ: Growth/decay with a fraction of the tumor undergoing cell death.
When more than one fit model may be selected based on the defined p-value, the fit model with the lowest Akaike Information Criterion (AIC) is considered in mint Lesion to automatically determine the "best fit" model for each patient based on their serial measurements. For additional flexibility in analysis, corrected AIC (AICc) can be applied as a correction for a small sample size.
Why Implement TGRM in Your Next Trial?
• Time as a Variable: Unlike RECIST, which requires fixed imaging intervals to avoid bias, TGRM treats time as a variable. This makes it ideal for Real-World Evidence (RWE) or retrospective analyses where assessment schedules may vary.
• Volumetric Precision: While the model works with diameters, mint Lesion’s volumetric segmentation may provide a more robust estimate of g, potentially reducing the sample size needed to detect treatment effects.
• Data Liquidity: mint Lesion facilitates data liquidity by allowing evaluated trial data and TGRM estimates to be exchanged between instances (mint-to-mint), supporting standardized data formats and consistent reporting across global research sites
Configurable Data Analysis Extraction
The g/d Tumor Growth Rate Export in mint Lesion is highly configurable. Users can filter data by specific lesion locations (e.g., focusing only on liver metastases), select p-value thresholds for model fits, or even run the analysis retrospectively on completed trials to extract new insights from historical data.
Learn more about the large-scale clinical analysis of g-values performed by the FDA that suggests g value may be a critical predictor of survival in our detailed summary of the research.
Discover how TGRM methodology can empower smaller patient datasets and earlier go/no-go decisions in drug development by accessing our comprehensive TGRM white paper.
1Justin N Malinou, Jiaxin Fan, Joyce Cheng, Yutao Gong, Yuan-Li Shen, Erin Larkins, An FDA analysis of the association of tumor growth rate, overall survival and progression-free survival in patients with metastatic NSCLC, The Oncologist, 2026.