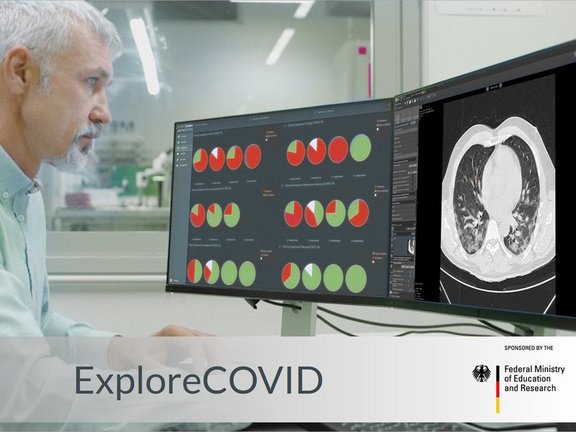
Funded by the German Federal Ministry of Education and Research, the ExploreCOVID project aims to analyze patient history and clinical as well as imaging data across multiple medical centers to identify standardizable diagnostic criteria. By doing so, a comprehensive database of structured data will be established which can be used for future epidemiological insights and the risk stratification of COVID-19 patients.
Current evidence indicates that chest CT imaging may be a valuable tool in the diagnosis, epidemiology, and therapy response evaluation of COVID-19 patients. It offers high sensitivity, short turnaround times, and wide availability. Therefore, chest CT may complement RT-PCR tests, especially in situations of unclear clinical presentation, such as a negative RT-PCR despite strong anamnestic evidence for COVID-19. More importantly, it offers opportunities to assess disease progression directly and may be a method of choice for therapy response assessment in upcoming trials of new therapeutic agents. However, to develop it into a proper instrument, a standardized quantitative approach is required. Additionally, multicentric cooperation is essential to generate a sufficiently large database with a consistently high data quality, which is crucial for modern data mining methods.
All centers involved in this project are provided with access to the COVID-19 functionality within the mint Lesion™ platform. The study centers can conduct structured reads on already available thoracic CT images, extract a wide range of image-based parameters (including radiomics parameters), associate them with other clinical and anamnestic data, and then transfer the resulting structured data to the project partners to identify the parameters relevant for the diagnosis and staging of COVID-19. Despite the very high number of different data sources, this approach guarantees that uniform and homogenous criteria are applied in the data extraction.
The results can be immediately implemented in the mint Lesion™ reading template and distributed worldwide for use in diagnostic procedures and therapy response assessments in clinical trials.
ExploreCOVID: An explorative cohort study to identify optimal CT imaging biomarkers in combination with clinical markers and PCR-RT for the diagnosis and therapy response assessment of COVID-19
Related Resources
Related Resources

Recording of RSNA 2019 AI theater presentation | Power food for AI
At this year’s RSNA, Mint Medical presented its AI approach to the audience at the AI theater. Tobias Gottmann and Aditya Jayaram highlighted how…

Data-driven healthcare through FIHR®-enabled interoperability
Nowadays, it is not unusual for healthcare systems to use numerous clinical applications from different vendors. Most tend to prefer flexibility for…
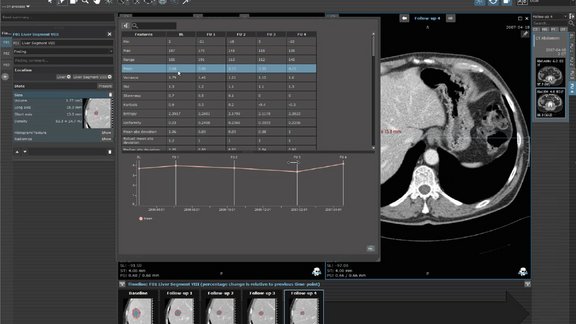
mint Lesion radiomics leverage precision medicine
The benefits that texture analysis and radiomics have shown already, and the improved availability of large data sets led to an increased interest in…