Our COVID-19 reading template is in use for two weeks, and its usage is multiplying. We are grateful for the close cooperation and feedback from existing and new mint Lesion™ users.
Our medical team went through all the available publications and created a reading template that can be used with ease by readers in your hospital or research center. As new papers and guidelines become available, we will update the reading template, retaining the structured data already collected.
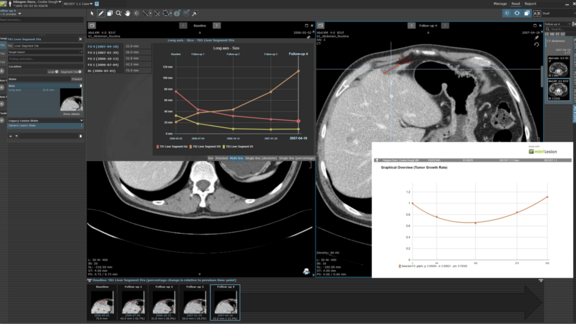
mint Lesion™ extracts all primary data - including radiomics features - from images, and immediately links any measured value to its context and further related data of clinical significance. Together with clinical endpoints, the mineable data is ready for real-time analytics and AI.
Let mint Lesion™ assist you in generating the data and creating the evidence that will enable us to tackle COVID-19 jointly.