For decades, oncology trials have relied on RECIST 1.1 to evaluate drug efficacy. However, these categorical "snapshot" evaluations do not always correlate perfectly with Overall Survival (OS) or Progression-Free Survival (PFS). A landmark FDA retrospective analysis of 10,495 patients across 22 randomized NSCLC clinical trials¹ is now suggesting a more precise kinetic approach that better correlates with survival: Tumor Growth Rate Modeling (TGRM) g-values.
The Power of the g Value
The FDA’s research focused on the growth rate constant (g), a volumetric measurement derived from mathematical models. The study’s most significant finding was that the g value is inversely associated with survival. Patients with lower g values—indicating slower tumor growth—showed significantly higher median OS and longer median PFS across all lines of therapy.
Key Insights from the FDA Publication
- Early Efficacy Signals: The g value can characterize the potential clinical activity of an agent before time-to-event endpoints like OS are reached.
- Therapy-Specific Kinetics: The analysis revealed that patients on targeted therapies (e.g., ALK or EGFR inhibitors) had the lowest g values compared to those on immunotherapy or chemotherapy.
- Predictive for Cytostatic Agents: TGRM is particularly useful for therapies with primarily cytostatic effects, where traditional response rates (ORR) might fail to capture the drug's true impact on growth inhibition.
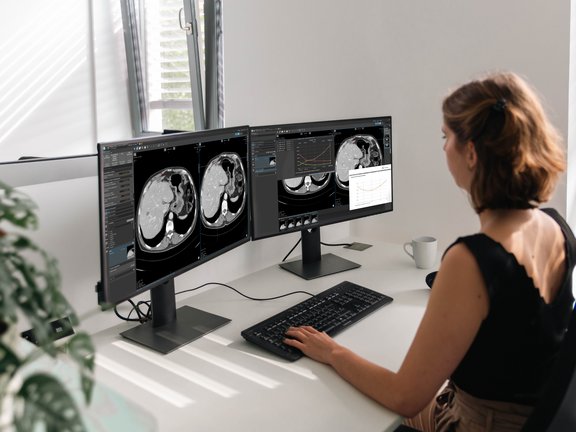
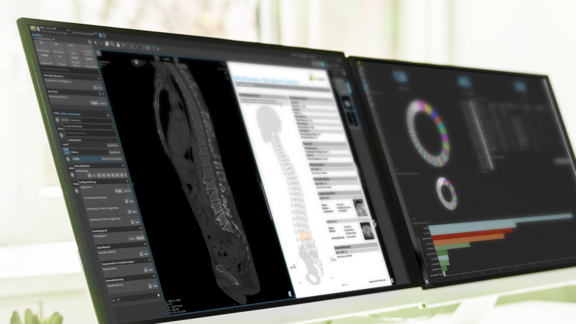
Implementation in mint Lesion
mint Lesion allows users to move beyond the broad binning of RECIST (like "Stable Disease") by quantifying these kinetics concurrently with standard assessments. By utilizing the same mathematical framework cited by the FDA, mint Lesion enables the concurrent extraction of g values, which exploratory research suggests may help characterize the potential antitumor activity of an agent early in the development process.
To understand how mint Lesion translates these mathematical models into a configurable clinical workflow, read our companion piece on the practical implementation of TGRM.
For a deeper dive into how TGRM overcomes the limitations of traditional response criteria to provide more predictive efficacy signals, download our full TGRM white paper.
1Justin N Malinou, Jiaxin Fan, Joyce Cheng, Yutao Gong, Yuan-Li Shen, Erin Larkins, An FDA analysis of the association of tumor growth rate, overall survival and progression-free survival in patients with metastatic NSCLC, The Oncologist, 2026.