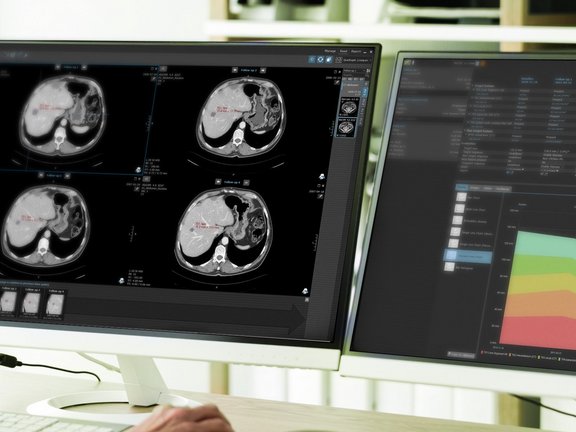

Benefit from Streamlined Data Exchange
Data generation
mint Lesion assures data uniformity by generating reliable, high-quality data – reported in a consistent...
Data liquidity
Data liquidity is a concept that allows previously gathered data to be used not just by the ones who...
Interoperability
Data from mint Lesion - or mintified data - can be used across systems, e.g., in any PACS or HIS...
Data generation
mint Lesion assures data uniformity by generating reliable, high-quality data – reported in a consistent, compliant, and structured way. Users of mint Lesion benefit from its ability to export every piece of information gathered within it, including images, into a mineable data format that allows for real-time, in-depth data analytics and learning opportunities.
Data liquidity
Manual transcription of data records including lesion measurements is prone to errors. Moreover, the creation process of such data records is irreproducible which makes it impossible to assess the integrity and quality of such records retrospectively. However, in the context of clinical trials, enabling data sharing amongst all parties is vital. Such data sharing should not be limited to the actual result values but should also cover all related raw data and intermediate data points.
Data Liquidity is a concept that allows previously gathered data to be used not just by the ones who collected it but also by other stakeholders. mint Lesion turns the concept of data liquidity into reality by leveraging the vast amount of data collected in clinical research and transforming this data into evidence that can be shared amongst all involved parties. This is achieved by communication of all associated raw data, intermediate data points, and further context information related to the process of data generation, such as user- and approval-information. Such data exports are semantically enriched and digitally signed to streamline the source data validation (SDV).
Interoperability
Data from mint Lesion - or mintified data - can be used across systems, e.g., in any PACS or HIS. Mintified DICOM images, including annotations, CRF assessments, protocol read template configurations applied in investigator sites and CRO-hosted mint Lesion instances can be exported directly to another mint Lesion instance. Various system interfaces are available to support the data flow necessary for the holistic treatment of patients.

Keep Pace with Novel and Modified Response Criteria
Rapid implementation of new criteria
New and revised response criteria are published faster than ever before. mint Lesion has a highly configurable...
Guidance on response criteria
mint Lesion provides feedback in the form of edit checks and algorithmic derivation of response assessments...
Criteria compliance and quality
mint Lesion guides its user through the reading process, ensuring that the requirements of the specific...
Rapid implementation of new criteria
New and revised response criteria are published faster than ever before. mint Lesion has a highly configurable and flexible rules base that keeps pace with the changing requirements and demands for new response criteria – allowing users to quickly apply modified or new guidelines.
Guidance on response criteria
mint Lesion guides its users through the reading process, ensuring that the requirements of the specific response criteria are met through continuous conformity checks. Should the user be unsure of their next step, they can rely on the helpful summary of the response criteria specifications anytime during or even before performing the read. Standardized CRF templates with integrated criteria overviews ensure user’s continuous training and minimization of errors.
Criteria compliance and quality
mint Lesion provides feedback in real-time in the form of edit checks and algorithmic derivation of response assessments. For automatically generated data points, such as the derived response category, the software provides users with a comprehensive "rationale" (e.g., target lesion SOD meets PR, new lesion causes PD, etc.)
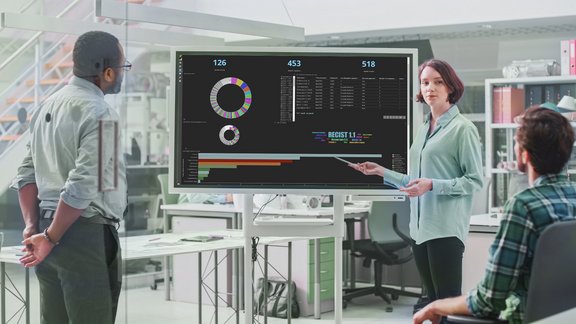
Improve Data Quality
Reduced variability
mint Lesion controls variability in read reporting by capturing the assessment data and deriving...
Improved trial efficiency, reduced data cleaning burden
The evolving complexity of clinical trials and response evaluations burgeons the need to ensure...
Data integrity and traceability
mint Lesion translates information from medical images, connecting it to other clinical data...
Reduced variability
mint Lesion controls variability in read reporting by capturing the assessment data and deriving the overall response consistently and correctly according to the criteria algorithm. This leads to improved consistency and compliance across sites and CROs using the same response criteria read templates in mint Lesion.
Improved trial efficiency, reduced data cleaning burden
The evolving complexity of clinical trials and response evaluations burgeons the need to ensure data quality and integrity in real-time. Such real-time quality control processes substantially improve trial efficiency and reduce the financial burden of quality control efforts as opposed to retrospective data cleanups and querying. mint Lesion provides feedback through conformity checks and algorithmic derivation of response assessment in real-time during each read. This minimizes criteria errors and missing information, and ensures a compliant, comprehensive read result.
Data quality, integrity and traceability
mint Lesion translates information from medical images, connecting it to other clinical data, then curates all this information into an accessible, mineable format. Furthermore, the data can be exported for external analysis, in various human- and machine-readable data formats, such as comma-separated values (.csv). In addition, all image annotations can be exchanged in open formats, such as NRRD or as DICOM-compliant annotations (DICOM RT, DICOM Seg. Surface Obj.) and integrated with other information systems.
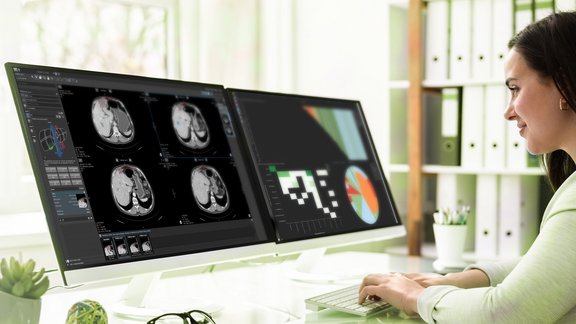
Generate Data in Real-Time
Start-up efficiency, minimal go-live time
mint Lesion comes with a large range of state-of-the-art response criteria, configurable to...
Real-time access to results
Evaluation rules are at the core of each response criteria. mint Lesion automates...
Integrated collection of radiomic features
Adherence to response criteria requirements is continuously monitored and deviations are reported to users...
Start-up efficiency, minimal go-live time
mint Lesion provides a compliant, verified, and comprehensive solution, ready to perform reads based on trial configuration requirements in a matter of days. Response analysis criteria, read paradigm workflows, conformity checks, notifications, and data exports are part of a comprehensive clinical trial service offered by Mint Medical. Reduced start-up time to go-live of productive reads provides tremendous financial benefit and ensures that real-time reads can be conducted as subjects enroll in your trial.
Real-time access to results
Data exports are available in multiple formats, including CSV, XML, PDF, and DICOM and in real-time as data are generated. Automated generation of structured best-in-class reports is highly customizable and allows information to be reproduced in graphs, snapshots and charts. mint Lesion reports can be sent directly to clinical sites for eligibility and confirmation of progression reads. Data transparency and liquidity ensure that you always have direct access to your data.
Integrated collection of radiomic features
One reason radiomics is ideally suited for collecting data in the context of treatment response evaluation is that large amounts of radiological data are routinely used for endpoint evaluation in clinical trials. Radiomics can be collected without interfering with the endpoint analysis of the trial, and the data can later be further analyzed along with genetic and clinical information. For example, a solid tumor trial can utilize RECIST 1.1 for the endpoint analysis of Progression Free Survival, while also collecting radiomic features in the background without impacting the efficacy evaluation.