Conduct Clinical Trials with Efficient Therapy Response Assessment Workflows
Always at the cutting edge of clinical research, mint Lesion embraces all important aspects of imaging-related cancer trials at site and central level. Being strongly rooted in both clinical radiology and pharmaceutical trials, we deeply understand your needs as a radiologist, trial manager, or principal investigator.
Clinical trial timelines from patient enrollment to data submission are continuously getting shorter in the global effort to bring new therapies to patients faster. New therapies with unique mechanisms of action can impact the evaluation of both imaging and clinical data assessments. These therapies require modified and novel response criteria to evaluate treatment effect, especially as clinical trials have shifted from indication-based to targeted approaches.
mint Lesion provides you with the efficiency of a verified read-ready platform for clinical trials, with structured reporting and site-specific workflows that support you in meeting the expectations of the trial sponsor.
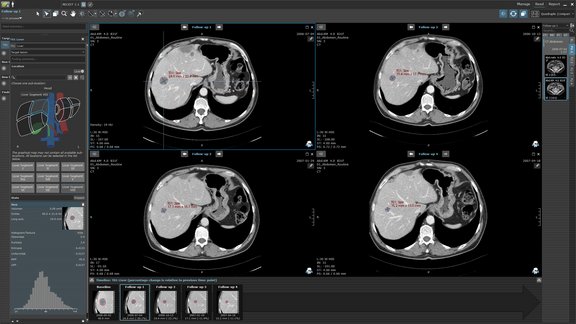
Collect High-Quality Data with Integrity
Built-in response criteria and flexible eCRF design
mint Lesion comes with a large range of state-of-the-art response criteria, configurable to...
Powerful rule logic
Evaluation rules are at the core of each response criteria. mint Lesion automates...
Conformity and completeness checks
Adherence to response criteria requirements is continuously monitored and deviations are reported to users...
Built-in response criteria and flexible eCRF design
mint Lesion™ comes with a large range of state-of-the-art response criteria, configurable to match common criteria variations required by typical clinical trials. New default criteria are continuously added as the scientific community refines drug evaluation standards.
Trial-specific imaging and non-imaging eCRFs and single data fields can be added for each patient visit or imaging exam. This includes both complementary eCRFs (such as patient eligibility forms) and the extension of built-in response criteria with additional questions (such as qualitative lesion description).
Powerful rule logic
Evaluation rules are at the core of each response criteria. mint Lesion™ automates the calculation of response classification conforming to the selected guidelines. Not only are the results like timepoint response or best overall response derived automatically, but they are also accompanied by a reasoning statement that can be included in reports, explaining why the software arrived at a certain conclusion. Automated results can be overridden at all levels, with a justification provided by the user. Built-in rules are designed to be exchangeable or tailored to the individual trial, if needed. Trial-specific rule logic can be defined on custom eCRF fields.
Conformity and completeness checks
Adherence to response criteria requirements is continuously monitored and deviations are reported to users. Readers can only submit an assessment once all mandatory conformity and completeness requirements are fulfilled – the exact requirements are configurable per trial.

Maximize Data Alignment
Innovative imaging workstation
A full-fledged radiology diagnostic workstation, mint Lesion integrates tumor response assessment right into...
Longitudinal data modeling
The mint Lesion data model is centered around the concept of a finding which, once identified...
Clinical integration
mint Lesion is fully integrated into the radiology and clinical IT infrastructure. Various options exist to...
Innovative imaging workstation
A full-fledged radiology diagnostic workstation, mint Lesion integrates tumor response assessment right into the radiological workflow. State-of-the-art image display and processing features are enhanced with tools optimized for the tumor evaluation process, such as short axis delineation or reference tissue measurements. When assessing single time points, images from all involved imaging modalities can be combined. This includes dedicated tools for advanced image analysis and display for example 3D+t-MRI or cross-vendor PET quantification, including SUV measurement and quantification of total glycolysis.
Longitudinal data modeling
The mint Lesion™ data model is centered around the concept of image observations which, once identified and described for an exam, can be re-evaluated at any future visit, both by arbitrary image measurements and eCRF questions specifically defined for it. This facilitates full comparability of data across any visit. Readers can easily access historic data of individual findings or entire exams by means of history charts, tables, and the patient timeline. Criteria logic, conformity checks, as well as reporting and data exports are all designed to take advantage of the inherent data longitudinality.
Clinical integration
mint Lesion™ can be integrated into the radiology and clinical IT infrastructure. Various options are available to automate the retrieval process of the required DICOM image data from PACS so that they are readily available for viewing when needed. Patient master data, order entries, textual and graphical reports, and structured data can be exchanged with other systems (e.g., RIS or HIS), using HL7 protocols, including HL7 FHIR. Central user and user role management is employed through seamless integration with hospital account management systems using the secure LDAP, SAML, or OAuth protocols, and an API enables deep integration with third-party systems, such as clinical trial management systems and databases.
Gain Insightful Data Beyond Tumor Size
Radiomics
All 2D ROI and volumetric measurements performed in mint Lesion can be used to calculate...
AI-ready structured data
The highly structured intrinsic data model of mint Lesion is suitable for AI and machine learning...
Data from routine reads
Clinical trial site reads are never isolated from actual patient care; the same image data is used...
Radiomics
All 2D ROI and volumetric measurements performed in mint Lesion™ can be used to calculate a radiomics footprint in compliance with the Imaging Biomarker Standardization Initiative (1st order and 2nd order features). As with any other quantitative value, radiomics results are directly accessible from within the User Interface (including charts showing their longitudinal development) and can be exported for individual patients or trial cohorts for statistical analysis.
AI-ready structured data
The highly structured intrinsic data model of mint Lesion™ is suitable for AI and machine learning applications. To ease compatibility with 3rd party systems, industry coding standards such as RadLex, SDTM, and SNOMED-CT are used for medical terminology and anatomical locations.
All data can be exported in file-based formats (e.g., CSV, XML) for further processing or statistical evaluation. Export capabilities include a sub-set of the CDISC standard for clinical trial data exchange, simplifying the data submission process where needed.
Data from routine reads
Clinical trial site reads are never isolated from actual patient care; the same image data is used for routine patient reporting, and critical findings identified during trial reads may directly affect patient care. As a diagnostic workstation with dedicated modules for patient screening, tumor staging and routine tumor monitoring, mint Lesion™ can be set up to bridge routine and trial assessments. For example, tumor measurements and classifications from a patient’s routine exam can be re-used as a basis for the clinical trial read later, and possibly be enhanced or re-classified as per specific needs of the trial protocol.

Streamline Your Trial Workflows
User roles and task assignment
mint Lesion comes with pre-configured user roles that represent the typical functions in...
Patient and trial management
Trial coordinator roles can create and manage clinical trials directly from within the...
Processing workflows
Workflows in mint Lesion are a powerful tool to streamline and automate the execution of...
User roles and task assignment
mint Lesion™ comes with pre-configured user roles that represent the typical functions in a clinical trials team, including trial coordinator, reader, reviewer, and sponsor. All roles are highly customizable in terms of the functions available and the data that is visible to them. Similarly, completely new user roles can be created by administrators.
All tasks (for example, tumor response assessments or auxiliary eCRFs) can be assigned to qualified readers and will appear on their individual worklist as soon as the task is ready for review. Alternatively, tasks can be made available to a pool of readers on a first-come-first-served basis.
Patient and trial management
Trial coordinator roles can create and manage clinical trials directly from within the mint Lesion™ User Interface. This comprises the definition of multiple trial arms (each with their own set of response criteria and eCRFs if needed), the definition of default assignees, adding patients to trials, and taking patients off trials. Patient records are either automatically generated by DICOM image import or created manually by the research coordinator prior to imaging.
When adding a patient to the trial, each available image study is automatically associated with the visit and can be immediately sent to radiological review. If needed, multiple image studies can be evaluated under the same visit, e.g., for multi-modality examinations. Patient records can be assigned a trial-specific subject ID for each trial the patient participates in.
Processing workflows
Workflows in mint Lesion™ are a powerful tool to streamline and automate the execution of your clinical trials and can be freely configured in agreement with site-specific requirements or SOPs – for each individual trial, if needed. Complex review workflows can be designed by simple product configuration. The system automatically executes or schedules pre-defined actions, such as data export or report generation, transmitting data to 3rd party systems, email notifications, automated re-assignment, and many more.
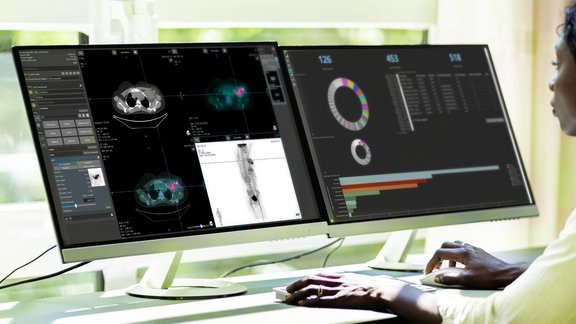
Explore Your Data from Different Perspectives
Concurrent evaluations
Trials can be modeled to require multiple parallel or sequential tumor evaluations using...
Analytics and monitoring dashboards
A dedicated dashboard application provides visual insights into trial data, allowing...
Reporting flavors
mint Lesion supports various human-readable reporting formats, ranging from highly visual...
Concurrent evaluations
Trials can be modeled to require multiple parallel or sequential tumor evaluations using trial arms. It is, therefore, easier to manage crossover trials, in which multiple subsequent therapies are administered and different response criteria and/or eCRFs are simultaneously evaluated. Measurements from the last exam preceding the line of therapy can be re-used in the baseline of the current therapy. Similarly, the measurements and findings created, for example, with RECIST 1.1 criteria, can be used (and re-classified) for a parallel iRECIST exam.
Analytics and monitoring dashboards
mint Analytics, an add-on of mint Lesion™, enables you to visualize all your data from one or multiple trials in graphs and charts. Using a configurable search engine, mint Analytics empowers you to interactively explore intriguing facets of an ongoing trial. Predefined, customized analysis dashboards can be used to quickly extract relevant exploratory statistics. The same application offers dedicated tools for easy monitoring of trial progress and performance measures.
Reporting flavors
mint Lesion™ supports various human-readable reporting formats, ranging from highly visual PDF reports tailored to tumor response assessment, to simple reporting snippets that can be used as building blocks in radiology reports. Generated reports can be automatically sent to PACS, RIS, HIS, or exported to network shares at the time of task completion. All reports are based on the entire case history and include longitudinal data for each data point.
Multiple reports can be generated for each exam to suit the particular needs of each recipient. For example, mint Lesion™ can be configured to generate two automatic reports per read: one for in-house purposes, with the original patient details and other clinically relevant data, and another one with a clinical trial subject ID for the sponsor/CRO data submission.





