Mrs. Kelie Luby will join Mint Medical’s team from January 2017 as new Vice President Clinical Trials Software and will be introduced at the upcoming RSNA congress in Chicago.
Kelie is an experienced medical scientist and communicator with more than 18 years of clinical trial experience, including 12 years in the Imaging Contract Research Organization (CRO) space. Based on the US East Coast, Kelie extends our expertise in medical imaging analysis, implementation of all standard as well as complex scientific assessment criteria used in oncology and other indications.
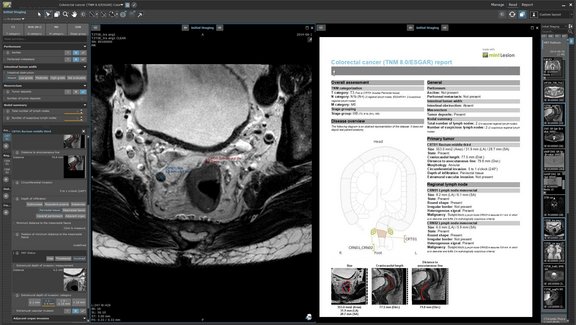
She has trained 100’s of medical experts, including radiologists, nuclear medicine physicians, oncologists and other specialists, on implementation of assessment criteria for central review of clinical trial data as well as investigators and other clinical site staff. She has hands on experience in several imaging software platforms including mint Lesion™ as well as other Imaging CRO proprietary platforms.