We are pleased to announce that we, as a medical software developer, have received the MDR certificate for the manufacture of class IIa and IIb devices.
The new EU Medical Device Regulation (EU 2017/745), abbreviated MDR, took effect at the end of May 2017. The aim of the MDR is to further increase patient safety by strengthening the transparency and traceability of medical devices through a dedicated product number and ensuring a high standard of quality, among other things.
The new regulation further increases the already demanding requirements for notified bodies, manufacturers, clinical data and market surveillance. The quality management system of Mint Medical was adapted to the significantly heightened requirements of the MDR and successfully audited by TÜV Süd Product Services. The certification confirms that mint Lesion™ meets the strict guidelines of the new regulation and will continue to serve our users as a trustworthy and safe medical device.

MDR certification: Another affirmation of the quality and safety of our software solution
Related Resources
Related Resources

Creating evidence to tackle COVID-19
Our COVID-19 reading template is in use for two weeks, and its usage is multiplying. We are grateful for the close cooperation and feedback from…
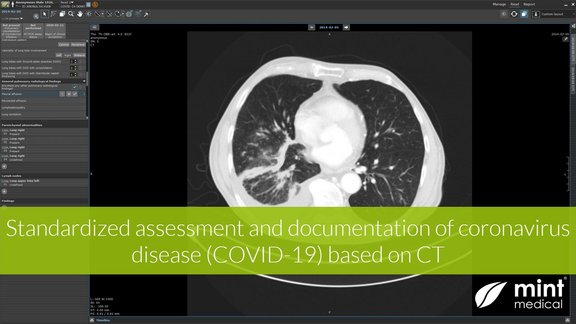
Mint Medical provides a reading template for the standardized assessment and documentation of COVID-19 disease (coronavirus) based on CT imaging
In the first week of March, we will make the relevant software functions available for all our current users at no cost. We plan to offer the COVID-19…
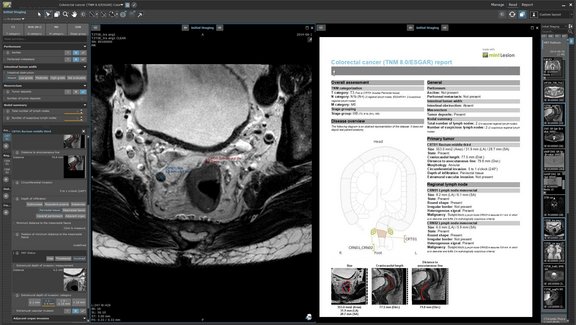
Clear, consistent, and complete documentation of imaging-derived information
The radiology report is expected to provide information that impacts life changing treatment decisions at every cancer care step in which imaging is…