Today, it is generally agreed that standardized language used in radiology reports improves communication with the treatment team and patients, with a huge impact on decision making and patient outcomes. Furthermore, using standardized forms to create consistent reports, helps to save time and effort while improving value.
Common Data Elements (CDE) are “data elements that are collected and stored uniformly across institutions and studies and are defined in a data dictionary”1. They are the result of a US-American standardization and harmonization initiative. Their development - both on the technical and on the medical side - is mainly driven by the National Institutes of Health (NIH) and its sub-organizations.
The use of CDEs leads to systematic, structured data collection, analysis, and share. It supports a seamless exchange of information between systems and facilitates analysis re-using collected data or aggregated data across studies, sites, and over time. In addition, it supports data retrieval using clinical terminology and has the potential to relate to electronic health records and routine healthcare ontologies.
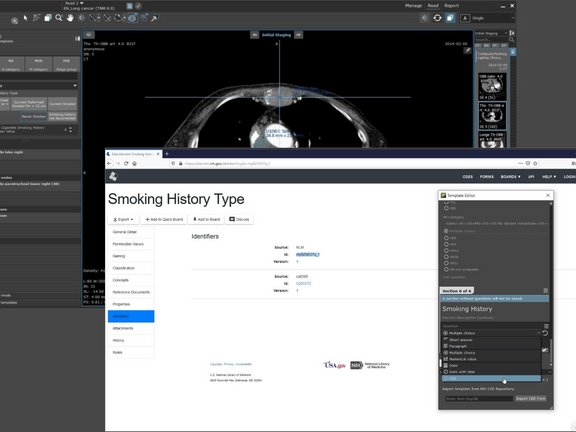
The National Institute of Cancer (NCI) is responsible for the development of CDEs in oncology and provides common metadata standards for all cancer data. The NCI and other organizations within the NIH are developing both individual CDEs and CDE forms, as CDEs combined into a questionnaire. For the application of CDEs in the mint Lesion™ software, two use cases were designed: The integration of an entire CDE questionnaire from the CDE repository and the import of individual CDEs into the mint Lesion™ TemplateEditor.
The import of the CDE forms allows users to add a ready-made questionnaire and to select and edit it like mint Lesion’s own questionnaires. By importing CDEs into the TemplateEditor, the user can create a questionnaire from CDE according to his own needs with minimal effort.
1Winget MD, Baron JA, Spitz MR et al. Development of common data elements: the experience of and recommendations from the early detection research network. Int J Med Inform 2003;70(1):41–48.